Brazilian scientists have achieved a revolutionary milestone in neurological medicine with the development of polylaminin, the first drug scientifically proven to regenerate damaged spinal cords.
Background and Discovery
The groundbreaking innovation is the result of 25 years of dedicated research at the Federal University of Rio de Janeiro. This sustained effort marks Brazil’s emergence as a global leader in regenerative medicine through consistent investments in biomedical innovation.
Polylaminin: A Hope for Recovery
Polylaminin is a unique drug designed to target the central nervous system and restore motor function after trauma. Its development signals a new era for spinal cord injury treatment, surpassing the limitations of existing methods.
Key Takeaways
- Decades of Research: Polylaminin represents 25 years of Brazilian research, showcasing the nation’s leadership in regenerative medicine.
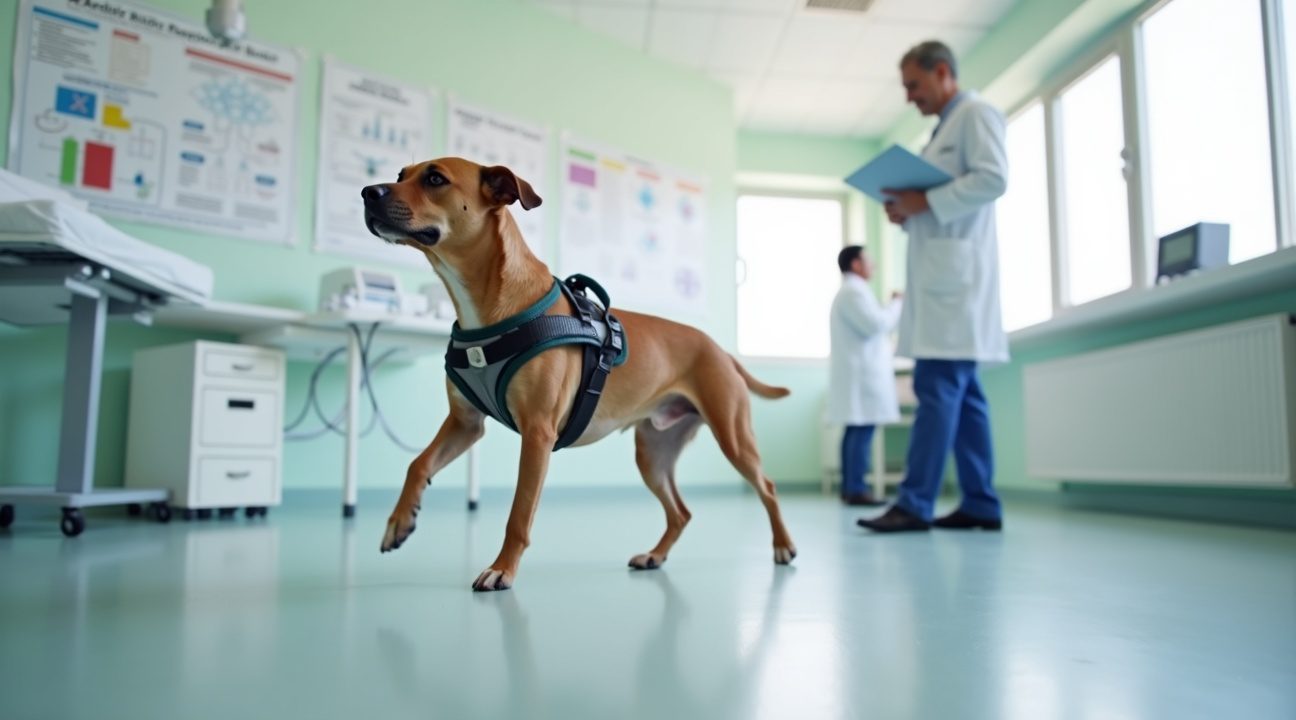
- Promising Preclinical Results: Trials on paraplegic dogs showed a 67% success rate, with four out of six regaining the ability to walk.
- Positive Human Trials: Though involving fewer than 10 patients, early human trials indicate varying degrees of neurological improvement, especially when administered during the acute injury phase.
- Exceptional Safety Profile: No serious side effects were reported in either animal or early human studies, distinguishing this treatment from risk-laden conventional options.
- Awaiting Regulatory Approval: Despite strong results, the treatment is still under evaluation, as Brazil’s national health regulator, Anvisa, has yet to approve it for clinical use.
Next Steps and Future Outlook
As further trials proceed and regulatory reviews continue, medical communities worldwide will be watching closely. Approval and broader access to polylaminin could change the future of rehabilitation for millions suffering from spinal injuries.
Brazilian Scientists Achieve Breakthrough: Dogs Walk Again After Spinal Cord Treatment
Brazilian researchers have achieved remarkable success with their polylaminin treatment in preclinical trials involving dogs with spinal cord injuries. This groundbreaking research demonstrates the drug’s potential to restore function in severely damaged nervous tissue.
Promising Results in Canine Studies
The preclinical studies involved six paraplegic dogs that received polylaminin treatment. The results were striking – four of the six animals regained their ability to walk after months of careful monitoring. These outcomes represent a significant advancement in spinal cord injury treatment, particularly because researchers observed no serious side effects throughout the extended follow-up period.
The success rate in these dog studies provides compelling evidence that polylaminin can facilitate meaningful recovery in cases of severe spinal cord damage. Each treated animal underwent comprehensive evaluation to track their progress, with researchers documenting improvements in motor function and overall mobility. This careful monitoring approach ensures that any potential adverse effects would be identified while confirming the treatment’s therapeutic benefits.
Early human trials under academic protocols have shown encouraging preliminary results, though the participant pool remains small with fewer than 10 individuals involved. The human experimental data reveals varying degrees of recovery among participants, ranging from minimal movement restoration to more extensive motor improvements. This variation suggests that individual factors may influence treatment outcomes, though the overall trend points toward positive therapeutic potential.
One particularly compelling case involves Bruno Drummond, whose treatment became well-documented due to the significant improvements he experienced. Drummond’s case stands out because he received polylaminin treatment immediately during the acute phase of his injury. His recovery trajectory highlighted something crucial about timing – early intervention appears to play a vital role in maximizing treatment effectiveness.
The distinction between acute and chronic spinal cord injury treatment timing has emerged as a critical factor in these studies. Patients who receive polylaminin during the acute phase, when inflammation and secondary damage are still developing, may experience better outcomes compared to those treated months or years after their initial injury. This finding could reshape how medical professionals approach spinal cord injury treatment protocols.
The absence of serious adverse effects in both animal and human studies represents another significant milestone. Traditional spinal cord injury treatments often carry substantial risks, making polylaminin’s safety profile particularly noteworthy. Researchers continue monitoring participants for any delayed reactions, but current data suggests the treatment is well-tolerated across different patient populations.
Recovery statistics from these early trials indicate that movement restoration varies considerably among participants. Some individuals experience subtle improvements in sensation or minimal motor function, while others achieve more dramatic gains in mobility and muscle control. Understanding these variations will be essential as researchers refine treatment protocols and identify optimal candidate criteria.
The gait recovery observed in the dog studies provides a tangible measure of treatment success. Scientific breakthroughs like this often begin with animal models before progressing to human applications, and the canine results suggest strong potential for similar outcomes in human patients.
These breakthrough results position Brazil at the forefront of regenerative medicine research. The polylaminin treatment represents 25 years of dedicated scientific work, with each study building upon previous discoveries to create this innovative therapeutic approach. The combination of safety and efficacy demonstrated in both animal and human studies suggests that this treatment could transform how medical professionals address spinal cord injuries.
Current research continues to explore:
- Optimal dosing protocols
- Treatment timing
- Patient selection criteria
Each new case provides additional data points that help researchers understand how to maximize polylaminin’s therapeutic potential while maintaining its impressive safety record.
A Quarter-Century Journey: From Placenta Protein to Regenerative Medicine
I’ve witnessed few scientific endeavors match the persistence and dedication that Brazilian researchers have shown in developing polylaminin. The Federal University of Rio de Janeiro (UFRJ) embarked on this ambitious project over 25 years ago, recognizing the potential of laminin protein found in human placenta to revolutionize spinal cord injury treatment.
The journey began with researchers identifying unique properties within placental tissue that could stimulate neural regeneration. This discovery marked Brazil’s entry into cutting-edge biomedical innovation, positioning the country as a serious player in regenerative medicine research. Scientists at UFRJ methodically studied how laminin proteins could be extracted and modified to create polylaminin, a synthetic version designed specifically for therapeutic applications.
Building Brazil’s Deeptech Infrastructure
The development of polylaminin required sustained institutional commitment that extended far beyond typical research timelines. FAPERJ, Rio de Janeiro’s state research foundation, provided continuous funding through multiple grant cycles, allowing researchers to pursue long-term studies essential for regenerative therapy development. This financial backing enabled Brazilian scientists to maintain focus on their spinal cord injury research without interruption, something that proves particularly challenging in biomedical innovation where results often take decades to materialize.
Research teams collaborated extensively with Cristalia, a Brazilian pharmaceutical manufacturer, bridging the critical gap between laboratory discovery and clinical application. This partnership demonstrated Brazil’s capacity to support deeptech initiatives from conception through manufacturing, creating a complete domestic pipeline for advanced medical treatments. The collaboration also ensured that intellectual property and manufacturing capabilities remained within Brazil, strengthening the country’s position in global biotechnology markets.
Scientific Breakthroughs and Clinical Applications
Polylaminin represents more than just a scientific achievement; it showcases Brazil’s ability to conduct world-class biomedical research over extended periods. The molecule works by providing scaffolding that supports nerve cell growth and reconnection in damaged spinal tissue. Research teams spent years optimizing the synthetic version to ensure it could effectively stimulate regeneration while maintaining safety standards required for human treatment.
Clinical trials revealed promising results for patients with various types of spinal cord injuries. The therapy showed particular effectiveness in cases where traditional treatments had failed to provide meaningful recovery. These outcomes validated the decades-long research investment and demonstrated that Brazilian institutions could compete with leading international research centers in developing breakthrough medical treatments.
The success of polylaminin has implications that extend beyond individual patient outcomes. Brazil’s achievement in regenerative medicine attracts international attention and investment, potentially leading to expanded research collaborations and technology transfer opportunities. This recognition helps establish Brazil as a destination for advanced medical research, similar to how NASA puts up trials for massive projects that capture global scientific interest.
The polylaminin project exemplifies how sustained research commitment can yield transformative results in biomedical innovation. UFRJ’s quarter-century dedication to understanding and developing this spinal cord injury treatment demonstrates that breakthrough discoveries require patience, persistent funding, and institutional vision. The successful collaboration between academic researchers, government funding agencies, and commercial manufacturers created a model for future Brazilian deeptech initiatives.
This achievement positions Brazil uniquely in regenerative medicine, with proven capabilities in both basic research and clinical application development. The polylaminin success story provides a foundation for additional investments in Brazilian biomedical research and establishes credibility for future ambitious projects in regenerative therapy and related fields.
How Brazil’s First Regenerative Drug Compares to Current Spinal Cord Treatments
Current treatments for spinal cord injuries operate on fundamentally different principles than Brazil’s groundbreaking regenerative approach. I examine how existing medications like pregabalin, riluzole, and 4-aminopyridine primarily focus on managing symptoms and providing neuroprotection rather than achieving true tissue regeneration.
Standard Pharmacological Interventions
Pregabalin stands as one of the most commonly prescribed medications for spinal cord injury patients, targeting neuropathic pain management rather than structural repair. Riluzole, originally developed for ALS treatment, offers neuroprotective benefits by modulating glutamate activity, yet it doesn’t restore damaged neural pathways. Similarly, 4-aminopyridine improves nerve conduction in damaged fibers but falls short of regenerating lost tissue.
These established treatments focus on symptom management and function preservation. Experimental strategies like PTEN inhibitors and histone acetylation promoters show promise in preclinical studies, promoting some degree of neural recovery through enhanced plasticity mechanisms. However, none of these approaches claim the comprehensive regenerative capabilities demonstrated in preclinical polylaminin models that inspired Brazil’s drug development.
Global Investment and Research Landscape
International investment in regenerative therapies has reached unprecedented levels, with billions of dollars flowing into spinal cord research initiatives. This massive financial commitment reflects the urgent medical need and commercial potential of breakthrough treatments. Startup companies and established pharmaceutical giants are racing to develop next-generation therapies that go beyond current limitations.
The competitive landscape includes various approaches:
- Stem cell therapies
- Bioengineered scaffolds
- Gene editing tools
- Neuroprotective devices
Each of these attempts to address different aspects of spinal cord damage. While many show promise in early trials, the field still lacks a clinically proven solution that can reliably regenerate damaged neural tissue. This gap explains why Brazil’s achievement generates such significant interest from both medical professionals and patients worldwide.
Brazil has positioned itself as a leader in advanced therapy development within Latin America. The country’s regulatory framework has authorized over 60 studies on biological or tissue-engineered products since 2018, demonstrating a commitment to cutting-edge medical research. This regulatory environment has enabled Brazilian researchers to pursue innovative approaches that might face greater barriers in other jurisdictions.
The nation’s clinical trial activity reflects a comprehensive strategy for advancing regenerative medicine. Brazilian institutions have cultivated expertise in complex biological therapies, creating an ecosystem that supports breakthrough research like the spinal cord regeneration project. This foundation explains how a 25-year development program could succeed where many international efforts have struggled.
Brazil’s regulatory landscape for advanced therapies provides a competitive advantage in bringing experimental treatments to clinical testing. The streamlined approval process for biological products has accelerated research timelines while maintaining safety standards. This approach contrasts with more conservative regulatory environments that may slow innovation in regenerative medicine.
The stark difference between Brazil’s regenerative drug and current treatments lies in their fundamental objectives. While existing medications aim to manage consequences of spinal cord injury, Brazil’s approach targets the underlying tissue damage itself. This represents a paradigm shift from symptom management to actual healing, potentially transforming how medical professionals approach spinal cord injuries.
Current international efforts often focus on incremental improvements to existing therapeutic strategies. Brazil’s quarter-century commitment to developing true regenerative capability demonstrates a different philosophy — one that prioritizes transformative outcomes over incremental progress. This distinction may explain why their approach succeeded where countless other attempts have fallen short of achieving genuine spinal cord regeneration. The implications extend beyond spinal cord injuries, as innovative research projects across various fields continue pushing boundaries of what’s medically possible.
https://www.youtube.com/watch?v=Ke5YBqB_sW8
The Regulatory Reality: Why This Treatment Isn’t Available Yet
Polylaminin faces significant regulatory hurdles before reaching patients. Brazil’s health regulator, Anvisa, hasn’t approved the treatment for general clinical use, keeping it confined to experimental and regulatory clinical trial phases. The path from laboratory breakthrough to accessible medicine requires extensive validation that can take years to complete.
Clinical Trial Requirements and Safety Validation
Large-scale, randomized, controlled multicenter trials remain the primary obstacle preventing Polylaminin’s approval. These studies must demonstrate both efficacy and safety across diverse patient populations, requiring hundreds or thousands of participants. Researchers must also determine optimal dosage protocols, precise timing for treatment administration, and effective rehabilitation strategies that maximize the drug’s regenerative potential.
The complexity extends beyond simple efficacy testing. Scientists need to:
- Establish which patients respond best to treatment
- Identify potential side effects
- Create standardized protocols for administration
This comprehensive approach ensures patient safety while building the evidence base Anvisa requires for approval.
Manufacturing and Quality Control Challenges
Production scale-up presents another major barrier to availability. Manufacturing Polylaminin requires sourcing placentas and maintaining strict biological safety standards throughout the process. Good manufacturing practices (GMP) compliance adds layers of complexity to production, demanding specialized facilities and rigorous quality control measures.
Current manufacturing capabilities can’t meet potential demand if approval occurs. Scaling production while maintaining consistent quality requires substantial investment in infrastructure and personnel training. Each batch must meet exact specifications, and contamination risks must be eliminated through multiple safety checkpoints.
Brazil has modernized its regulatory framework through RDCs 2018/2021 and Law 14.874/2024, creating clearer pathways for advanced therapies. However, technical standards specific to regenerative treatments like Polylaminin continue developing. Pricing and reimbursement frameworks also need establishment before widespread access becomes possible.
These regulatory hurdles, while necessary for patient protection, create significant delays. The treatment that took 25 years to develop now faces additional years of regulatory review. Each requirement serves an important purpose, but the cumulative effect keeps this potential breakthrough from reaching patients who might benefit immediately.
The regulatory process reflects the high stakes involved in spinal cord treatments. Unlike other medical innovations that might pose limited risks, regenerative therapies could have profound long-term effects on patients’ neurological function. Anvisa’s careful approach protects patients while ensuring that approved treatments meet the highest safety and efficacy standards.

Transforming Brazilian Pharma: From Generics to Groundbreaking Innovation
The polylaminin breakthrough represents far more than a single scientific achievement—it signals a fundamental transformation in Brazil’s pharmaceutical landscape. For decades, Brazilian companies concentrated primarily on generic drug production, but this spinal cord regeneration project demonstrates how the industry has evolved into a serious contender in proprietary biologics and cutting-edge research.
Leading pharmaceutical companies like Cristalia, Biomm, Blau, and Biolab have embraced this strategic pivot, investing heavily in research and development that goes beyond traditional manufacturing. These organizations recognize that sustainable growth requires innovation rather than simply replicating existing treatments. The success of the polylaminin project provides a compelling model for other Brazilian firms considering similar transitions into specialized therapeutics.
Building the Infrastructure for Innovation
Several key developments must continue to support Brazil’s pharmaceutical transformation:
- Expansion of public-private partnerships that leverage government resources with industry expertise
- Development of specialized infrastructure including state-of-the-art GMP labs and comprehensive clinical research networks
- Strategic fostering of deeptech collaborations and startup partnerships to accelerate innovation cycles
- Implementation of robust translational medicine programs that bridge laboratory discoveries with clinical applications
- Creation of comprehensive ecosystem building initiatives that connect researchers, manufacturers, and healthcare providers
The infrastructure requirements for advanced biologics differ significantly from generic production facilities. Companies must invest in sophisticated manufacturing capabilities while simultaneously developing the clinical expertise necessary to navigate complex regulatory pathways. This dual investment strategy has proven essential for organizations serious about competing in the global biologics market.
Long-term vision emerges as perhaps the most critical factor in sustaining Brazil’s pharmaceutical evolution. The polylaminin project’s 25-year development timeline demonstrates that breakthrough innovations require sustained commitment and patience. Companies and government agencies must resist the temptation to seek immediate returns when building innovation ecosystems that can compete internationally.
Adequate funding mechanisms present another crucial element for success. Traditional pharmaceutical funding models often prove insufficient for the extended development cycles required by revolutionary treatments. Brazil has successfully implemented hybrid funding approaches that combine government investment, private capital, and international partnerships to support ambitious research projects.
Regulatory agility has become increasingly important as Brazilian pharmaceutical companies venture into uncharted therapeutic territories. The traditional regulatory framework designed for generic approvals requires adaptation to handle novel biologics and regenerative medicines. Recent regulatory improvements have streamlined approval processes while maintaining safety standards, creating an environment where innovative treatments can reach patients more efficiently.
The polylaminin success story illustrates how Brazilian pharma companies can build self-sufficient innovation ecosystems that reduce dependence on foreign technology transfers. This independence allows companies to pursue research directions that align with local health priorities while maintaining the flexibility to address global therapeutic needs.
Deeptech collaborations have proven particularly valuable in accelerating innovation cycles. By partnering with specialized technology companies and research institutions, pharmaceutical firms gain access to cutting-edge tools and methodologies without the need for massive internal investments. These partnerships often yield unexpected breakthroughs that advance multiple research programs simultaneously.
The transformation from generic manufacturing to innovation leadership requires companies to develop entirely new competencies. Research and development teams must expand beyond traditional chemistry into areas like cell biology, tissue engineering, and regenerative medicine. This expansion demands significant investment in talent acquisition and training programs.
International recognition of Brazilian pharmaceutical innovation has grown substantially following the polylaminin breakthrough. Global pharmaceutical companies increasingly view Brazilian firms as potential partners rather than simply manufacturing contractors. This shift in perception opens new opportunities for collaborative development projects and technology licensing agreements.
The success of Brazil’s pharmaceutical transformation ultimately depends on maintaining momentum across multiple fronts simultaneously. Companies must continue investing in research capabilities while government agencies work to enhance regulatory frameworks and funding mechanisms. This coordinated approach ensures that individual breakthroughs like polylaminin become part of a sustained innovation ecosystem rather than isolated achievements.
What It Takes to Bring Regenerative Medicine from Lab to Patients
The journey from laboratory breakthrough to clinical reality presents unique challenges that require careful planning and substantial resources. Brazil’s development of Polylaminin exemplifies the complex translational pathway that regenerative therapies must traverse before reaching patients who desperately need them.
Overcoming Critical Implementation Barriers
Clinical adoption of regenerative medicine faces several interconnected obstacles that researchers and regulatory bodies must address systematically.
Key challenges include:
- Clinical Trials: Establishing safety and efficacy through comprehensive clinical trials involving diverse patient populations. Polylaminin’s developers spent decades creating proper protocols and gathering data to meet international regulatory standards.
- Manufacturing Standards: Ensuring scalability and consistency through specialized facilities and quality control measures. Certified manufacturing processes were necessary in Brazil to accommodate increased production volumes.
- Infrastructure: Developing infrastructure for the production, storage, and distribution of complex treatments, which many healthcare systems find difficult to support.
- Economic Models: Evaluating long-term cost-effectiveness to balance high upfront costs with potential reductions in lifetime care expenses.
Brazil’s implementation of Polylaminin offers guidance on how to create sustainable medical breakthroughs by designing payment models that ensure both innovation and access.
Risk management is also vital due to the novel mechanisms found in regenerative therapies. Post-market surveillance plays a critical role in tracking long-term patient outcomes to detect unexpected side effects. Brazil’s regulatory system adapted to collect extended safety data while still providing timely access to treatment.
Integrating regenerative medicine into healthcare systems also demands targeted physician education. Brazil’s solution involved establishing training programs for medical professionals to understand how to administer and monitor regenerative treatments properly.
The success of Polylaminin was fueled by robust collaboration between academic institutions and industry. Brazil’s case highlights how sustained scientific investment and patient-focused innovation can advance therapeutic development. This is akin to how innovation projects in other sectors require similar dedication and long-term vision.
Adapting regulatory frameworks is essential to address the distinct characteristics of regenerative medicine. Traditional drug approval mechanisms may not suffice for therapies that activate regenerative processes in the body. Brazil had to create new evaluation criteria that maintained safety while enabling innovation.
Furthermore, international collaboration is crucial as regenerative therapies strive for global impact. Brazil’s journey through Polylaminin’s development offers a blueprint other nations can adopt. The exchange of knowledge on clinical protocols, regulatory standards, and manufacturing techniques accelerates the worldwide progress of these advanced therapies.
Ultimately, Polylaminin reflects Brazil’s strategic vision and capability in biotechnology. Its success proves that with long-term commitment and coordinated stakeholder involvement, even emerging economies can shape high-tech medical innovations and influence global standards in healthcare.
This model serves as a guide for other regenerative therapies developing from academic and industrial partnerships. Success lies in aligning goals among researchers, regulators, healthcare providers, and financiers. Brazil’s example illustrates that with deliberate strategy and enduring effort, obstacles on the path from discovery to delivery can be surmounted.
Sources:
IBIS.bio, “Polylaminin and the Challenge of Elevating Brazilian Biomedical Innovation Beyond the Promise”
PMC, “Molecular approaches for spinal cord injury treatment”
Jornal Nacional (Ensaio Clinicos), “Brazilian scientists use placenta protein to restore some movement in dogs and humans with spinal cord injuries”


