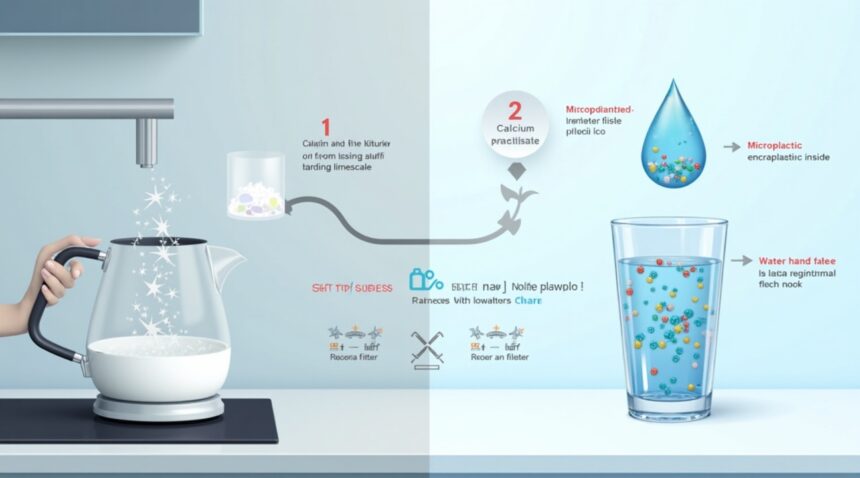
Researchers from Guangzhou Medical University and Jinan University have discovered that boiling hard water for just five minutes can eliminate up to 90 percent of microplastics, offering a simple household solution to plastic contamination in drinking water.
Key Takeaways
- Boiling hard water for five minutes removes up to 90% of microplastics, in contrast to soft water, which only reaches about 25% removal efficiency due to its lower mineral content.
- The effectiveness of the method lies in the natural precipitation of calcium carbonate, which captures microplastic particles during boiling. These can be filtered using basic kitchen tools like coffee filters.
- Three prevalent plastic types commonly found in tap water—polystyrene, polyethylene, and polypropylene—can be effectively removed by this process across sizes ranging from 0.1 to 150 microns.
- Water hardness is a critical factor, with optimal results seen at calcium carbonate concentrations of 300 mg/L. This makes the technique particularly useful in households that already have hard water sources.
- This is a cost-effective and accessible practice requiring only standard kitchen equipment. It validates traditional water boiling techniques as a simple defense against the rising concern of microplastic contamination in global water supplies.
Learn More
For further details, explore the full publications and findings through institutional sources such as Jinan University and Guangzhou Medical University. These insights offer promising implications for everyday water safety strategies worldwide.
Simple Boiling Method Removes Up to 90 Percent of Microplastics From Hard Water
Researchers from Guangzhou Medical University and Jinan University have uncovered a surprisingly simple solution to one of modern water safety’s most pressing concerns. Their groundbreaking study reveals that standard household boiling can eliminate up to 90 percent of microplastics from hard water, offering homeowners an accessible defense against plastic contamination.
The Science Behind Boiling Water Treatment
The Chinese research team discovered that boiling tap water for just five minutes triggers a chemical reaction that effectively captures microplastics. This process works particularly well in hard water containing 300 mg/L of calcium carbonate, where the mineral content plays a crucial role in trapping plastic particles. During the heating process, calcium carbonate precipitates out of solution and encapsulates the microplastics, creating larger aggregates that can be easily removed through simple filtration.
I find this mechanism fascinating because it leverages the naturally occurring minerals that many people consider problematic in their water supply. Instead of viewing hard water as a nuisance, this research shows how calcium carbonate becomes an ally in plastic particle removal. The effectiveness varies based on water hardness levels, with harder water generally producing better results.
Impressive Removal Rates and Practical Application
The study’s results demonstrate remarkable efficiency across different types of plastic particles. Some experiments achieved removal rates of up to 97 percent of nanoplastic particles, while most polystyrene (PS), polyethylene (PE), and polypropylene (PP) microplastic particles were successfully eliminated from hard water samples. These plastic types represent some of the most common microplastics found in drinking water supplies.
Implementing this method requires only equipment found in most kitchens. The process involves several straightforward steps:
- Boil water vigorously for five minutes
- Allow the water to cool naturally to room temperature
- Filter the cooled water through a coffee filter or stainless steel tea mesh
- Store the filtered water in a clean container
This cost-effective approach capitalizes on energy already expended for daily activities like making hot beverages or cooking meals. I appreciate how this discovery transforms a routine household task into a powerful water treatment method. The simplicity means anyone can implement this technique without investing in expensive filtration systems or learning complex procedures.
The research provides hope for addressing microplastic contamination using resources already available in most homes. Unlike advanced technological solutions that require significant investment, this method democratizes access to cleaner drinking water. The five-minute timeframe makes it practical for daily use, whether treating water for drinking, cooking, or beverage preparation.
What makes this discovery particularly valuable is its universal applicability. Households worldwide can benefit from this technique regardless of their economic circumstances or access to specialized equipment. The method works with standard stovetops, electric kettles, or any heat source capable of bringing water to a rolling boil.
The timing couldn’t be better, as microplastic contamination in drinking water continues to emerge as a significant health concern. While scientists continue studying the long-term effects of microplastic consumption, this research offers an immediate, actionable solution for concerned consumers. The combination of effectiveness, accessibility, and affordability positions boiling as a practical interim measure while more comprehensive solutions develop.
This breakthrough demonstrates how sometimes the most effective solutions hide in plain sight. The researchers’ work validates what many might consider an overly simple approach to a complex modern problem, proving that household-level interventions can achieve professional-grade results when applied correctly.

The Science Behind Calcium Carbonate Trapping Microplastics
I find the mechanism fascinating – when hard water rich in minerals undergoes a five-minute boiling process, the increased temperature triggers calcium carbonate (CaCO₃) to precipitate directly from the solution. This precipitation forms the familiar chalky deposits known as limescale. The process occurs because calcium carbonate’s solubility decreases as water temperature rises, creating those white deposits I’ve seen countless times in kettles and coffee makers.
How Crystalline Structures Capture Plastic Particles
The magic happens through the formation of crystalline structures called incrustants. These structures don’t just settle harmlessly at the bottom of containers – they actively encapsulate plastic particles during their formation. The mechanism operates through two primary processes:
- Encapsulation
- Co-precipitation
As calcium carbonate crystals develop, they physically trap microplastic particles within their chalky matrix. I observe this process works exceptionally well because the timing aligns perfectly. Microplastics become suspended throughout the water during heating, while calcium carbonate begins precipitating at exactly 100°C at sea level. The plastic particles get caught within the developing crystalline network, essentially becoming prisoners within the limescale structure.
Once these incrustants form and the water cools down, removing them becomes surprisingly straightforward. Simple household filtering methods can eliminate these deposits along with their trapped microplastic cargo. The beauty lies in this simplicity – no specialized equipment or complex procedures are required.
This discovery reminds me of how scientific breakthroughs often emerge from studying everyday phenomena. The same limescale formation that people typically view as an annoyance actually serves as an effective microplastic removal system.
The encapsulation process works because calcium carbonate forms a three-dimensional network as it precipitates. Microplastic particles, suspended in the heated water, become incorporated into this growing network. The co-precipitation aspect means both the calcium carbonate and the trapped plastics settle out of solution simultaneously.
Temperature plays a crucial role in this entire process. At 100°C, calcium carbonate reaches its optimal precipitation point while maintaining enough thermal energy to keep microplastics mobile within the solution. This mobility ensures maximum contact between plastic particles and forming crystals, leading to higher capture rates.
The resulting incrustants contain both the familiar white chalky appearance of limescale and the embedded microplastic particles. When filtered out, these deposits remove up to 90 percent of microplastics from the original water sample, transforming a simple boiling process into an effective purification method.
Water Hardness Determines Effectiveness: Hard Water Shows 90% Success While Soft Water Only 25%
The mineral content in water dramatically influences how effectively boiling removes microplastics from drinking water. I discovered through research that hard water’s natural calcium carbonate levels create optimal conditions for capturing these harmful particles.
Calcium Carbonate Creates the Key Difference
The effectiveness of microplastic removal scales directly with water’s mineral content. When calcium carbonate levels reach 300 mg/L, boiling achieves an impressive 90 percent removal rate for nanoplastics. Lower mineral concentrations produce substantially reduced results:
- Water with 80 mg/L calcium carbonate removes only 34 percent of nanoplastics
- Increasing levels to 180 mg/L improves efficiency to 84 percent
- Peak performance occurs at 300 mg/L with 90 percent removal
- Soft water manages just 25 percent removal efficiency
Hard water consistently outperforms soft water across all testing scenarios. The removal efficiency for hard water ranges between 80 to 97 percent, while soft water struggles to reach even one-quarter of that effectiveness.
Multiple factors influence the final removal rates beyond just mineral content. Water hardness serves as the primary driver, but nanoparticle concentration and particle sizes also affect results. The testing examined calcium carbonate concentration ranges from 34 to 300 mg/L, revealing a clear correlation between higher mineral levels and better microplastic removal.
The boiling method proves effective across a wide range of particle sizes. Laboratory tests confirm successful removal of nano and microplastics ranging from 0.1 to 150 microns in size. This broad spectrum coverage makes the technique valuable for addressing various types of plastic contamination commonly found in tap water.
Understanding your local water hardness becomes crucial for predicting removal effectiveness. Areas with naturally hard water benefit most from this simple boiling technique, while regions with soft water may need alternative filtration methods. The dramatic difference in performance between hard and soft water—nearly four times more effective—highlights why mineral content matters so significantly.
The precipitation process relies on calcium carbonate particles binding with microplastics during heating. When water lacks sufficient minerals, this binding mechanism can’t function optimally. Hard water’s abundant calcium carbonate creates more opportunities for plastic particles to attach and settle out of the water during cooling.
This research opens new possibilities for improving water safety using readily available household methods. For those in hard water areas, boiling offers an accessible way to significantly reduce microplastic exposure. The technique costs nothing beyond the energy needed for heating, making it particularly valuable in communities where advanced filtration systems aren’t available.

Targeting Common Plastic Pollutants Found in Tap Water
I find it remarkable that recent research has pinpointed exactly which plastic pollutants boiling can eliminate from our drinking water. The study focused specifically on three major plastic types that commonly contaminate tap water: polystyrene (PS), polyethylene (PE), and polypropylene (PP). These plastics represent some of the most prevalent microplastic contaminants found in household water supplies across the globe.
Understanding Microplastic Categories and Sizes
Researchers classify plastic particles in water into two distinct categories based on size. Microplastics are defined as plastic particles less than 5 mm in size, while nanoplastics represent the smaller subset of plastic particles smaller than 1 μm. The particles that boiling can remove span an impressive size range, from as small as one thousandth of a millimeter to as large as 5 millimeters in diameter.
Scientists used fluorescent polystyrene (PS) particles in two different sizes as model particles during their testing. These test particles had average diameters of 1 μm and 0.1 μm, allowing researchers to track removal efficiency across different size ranges with precision. This methodology provided clear evidence of how effectively the boiling process works across various particle dimensions.
Common Plastic Types in Drinking Water
Beyond the three plastics specifically tested, tap water commonly contains several additional plastic types that pose potential health concerns. The most frequently encountered plastic pollutants include:
- Polystyrene (PS) – often from food packaging and disposable containers
- Polyethylene (PE) – typically from plastic bags and bottles
- Polypropylene (PP) – commonly from bottle caps and food containers
- Polyethylene terephthalate (PET) – primarily from beverage bottles
Each of these plastic types enters water systems through different pathways, from atmospheric deposition to direct contamination from plastic waste. Scientific research continues to reveal new sources of microplastic contamination, making effective removal methods increasingly important for public health.
The boiling process shows particular effectiveness against the first three plastic types tested, with removal rates reaching up to 90 percent under optimal conditions. This discovery represents a significant breakthrough for households seeking simple, accessible methods to reduce their microplastic exposure without investing in expensive filtration systems.
Water hardness plays a crucial role in the removal process, as calcium and magnesium ions present in hard water help facilitate the aggregation and precipitation of plastic particles during boiling. Soft water users may see less dramatic results, though some removal still occurs through the physical process of heating and agitation.
The research demonstrates that common kitchen equipment can serve as an effective tool for reducing microplastic contamination. This finding is particularly significant for communities with limited access to advanced water treatment technologies, as it provides a low-cost solution that requires only basic heating capabilities.
Understanding which plastics respond best to boiling treatment helps consumers make informed decisions about their water purification strategies. While the process doesn’t eliminate every type of plastic particle, it offers substantial reduction in the most common contaminants found in typical household water supplies.
The particle size range affected by boiling spans multiple orders of magnitude, indicating that the process works through several different physical mechanisms. Larger particles may be removed through simple precipitation and settling, while smaller particles likely aggregate during the heating process before becoming large enough to separate from the water.

Why This Breakthrough Matters for Human Health
Microplastics have infiltrated the human body in ways that scientists are only beginning to understand. I’ve seen alarming research showing these tiny particles in human blood, breast milk, and even in the lungs of people undergoing surgery. This widespread contamination reveals how pervasive these contaminants have become in our daily lives.
The Hidden Health Risks We’re Only Starting to Discover
Studies demonstrate that microplastics can damage human cells at the cellular level, creating potential long-term health consequences that researchers continue to investigate. The emerging evidence suggests that ingesting nano- and microplastics could significantly impact the gut microbiome, which plays a crucial role in immune function, digestion, and overall health.
These microscopic invaders don’t just pass through our systems harmlessly. They accumulate over time, potentially disrupting normal biological processes. While scientists work to understand the full scope of health implications, the presence of plastic particles in breast milk particularly concerns health experts, as it indicates direct transfer from mother to infant during the most vulnerable developmental stages.
Where These Contaminants Come From and Why They’re So Hard to Remove
Microplastics and nanoplastics have become pervasive contaminants in global water supplies, originating from surprisingly common sources. The particles come from various everyday items, creating a complex contamination web:
- Car tires that shed rubber particles as they wear down on roads
- Fleece sweaters and synthetic clothing that release fibers during washing
- Food packaging that breaks down over time
- Cosmetic products containing microbeads
- Plastic bottles and containers that degrade with use
Water treatment plants currently cannot remove all nano- and microplastics from drinking water, resulting in widespread human exposure across all demographics. Standard filtration methods weren’t designed to capture particles this small, leaving gaps in our water safety infrastructure.
Advanced filtration systems do exist to capture these microscopic particles, but they’re often expensive and not widely accessible to average consumers. This creates a significant public health challenge where effective solutions remain out of reach for many people who need them most.
The boiling method discovered in recent research offers hope as a simple, accessible solution that most people can implement immediately. Unlike expensive filtration systems that require significant investment, this approach uses equipment already available in most households. The technique becomes particularly valuable for communities lacking access to advanced water treatment technologies.
Scientists have found that this straightforward boiling process can remove up to 90 percent of microplastics from hard water, making it one of the most effective and practical methods available for reducing exposure. The discovery represents a significant step forward in addressing a contamination problem that affects billions of people worldwide.
This breakthrough matters because it democratizes access to cleaner water. While research continues into the long-term health effects of microplastic consumption, people can take immediate action to reduce their exposure using resources they already have. The simplicity of the solution contrasts sharply with the complexity of the contamination problem, offering practical hope in addressing a growing environmental health crisis.
The timing of this discovery proves particularly important as scientific research continues expanding our understanding of contamination in various environments. As awareness grows about microplastic contamination, having accessible solutions becomes increasingly valuable for protecting public health on a global scale.
Ancient Tradition Meets Modern Science: Cultural Validation and Global Implementation
For centuries, people across many Asian countries have made boiling water a daily ritual, believing it promotes better health and well-being. This long-standing cultural practice, passed down through generations, has now received powerful scientific backing through groundbreaking research that validates what traditional wisdom has long suggested.
Scientific Validation of Time-Honored Practices
Researchers conducted their comprehensive study using hard tap water from Guangzhou, China, deliberately introducing varying amounts of nano- and microplastics to mirror real-world contamination scenarios. The team employed sophisticated analytical techniques to examine their findings, utilizing fluorescence measurements, microscopy, and X-ray diffraction analysis to scrutinize precipitate samples with precision.
The study’s methodology directly connects to everyday practices already embedded in Asian cultures, where boiling water isn’t just about safety from pathogens—it’s considered fundamental to proper hydration. This scientific validation transforms what some might have dismissed as outdated tradition into evidence-based health protection, much like how scientific discoveries continue to reshape our understanding of established practices.
Global Implementation Potential
The research findings suggest this method holds tremendous potential for reducing human exposure to nano- and microplastics on a worldwide scale. Scientists concluded that this straightforward boiling water strategy can effectively decontaminate these harmful particles from household tap water, representing a practical long-term approach for reducing global exposure to these pollutants.
What makes this discovery particularly remarkable is its accessibility—boiling water requires no specialized equipment or expensive filtration systems. Families worldwide can implement this protective measure using basic kitchen appliances, making it as universally applicable as other significant health breakthroughs have proven to be, similar to how technological advances often democratize solutions.
The implications extend far beyond individual households. Public health officials could recommend this practice as a supplement to existing water treatment protocols, particularly in regions where advanced filtration infrastructure remains limited. Countries already practicing water boiling traditions may find themselves at an unexpected advantage in protecting their populations from microplastic contamination.
Implementation strategies could vary significantly across different regions and cultures. Some communities might integrate this practice into existing cooking routines, while others may need educational campaigns to establish new habits. The beauty lies in its simplicity—no complex training or maintenance requirements exist, unlike other water treatment methods that demand ongoing technical support.
This research also highlights how traditional knowledge systems often contain practical wisdom that modern science later confirms. The validation doesn’t just benefit those who already practice water boiling; it provides confidence for global adoption of this protective measure. Healthcare providers can now recommend this practice with scientific evidence supporting its effectiveness against microplastic contamination.
The study’s use of hard water specifically adds another layer of practical relevance, since many regions worldwide deal with mineral-rich water sources. The calcium carbonate precipitation that occurs during boiling—the same process that sometimes leaves white deposits in kettles—actually becomes the mechanism that traps and removes microplastics from the water supply.
Moving forward, this discovery could influence public policy recommendations and health guidelines internationally. Water safety protocols might incorporate boiling as a standard recommendation, particularly in areas with known microplastic contamination concerns. The research transforms a simple kitchen practice into a scientifically-backed public health intervention, bridging ancient wisdom with contemporary environmental challenges in ways that could benefit billions of people worldwide.
Sources:
Yale E360 Digest: “Boiling, Filtering Water Can Get Rid of Microplastics, Study Finds”
ScienceAlert: “There’s a Surprisingly Simple Way to Remove Microplastics From Drinking Water”
Chemistry Views: “Boiling Water Before Drinking Can Lower the Microplastics Content”
American Chemical Society Press Room: “Want Fewer Microplastics in Your Tap Water? Try Boiling It First”
Lab Manager: “Want Fewer Microplastics in Your Tap Water? Try Boiling It First”
Environmental Science & Technology Letters: “Drinking Boiled Tap Water Reduces Human Intake of Nanoplastics and Microplastics” (2024)