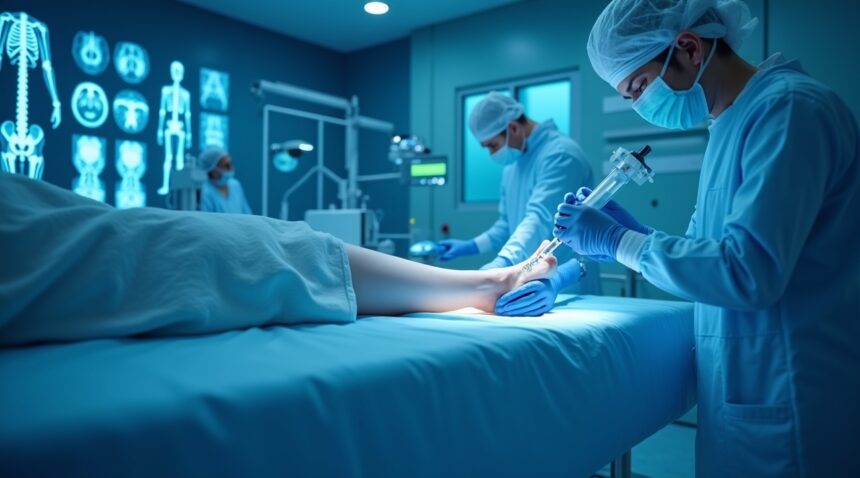
German scientists at the Fraunhofer Institute for Interfacial Engineering and Biotechnology have created a groundbreaking injectable collagen gel that regenerates cartilage tissue within minutes.
This innovative gel offers a minimally invasive alternative to traditional joint surgeries, forming a biodegradable scaffold upon injection that attracts the body’s own stem cells. As this scaffold supports the regrowth of natural cartilage, it safely dissolves without leaving behind foreign material. MRI scans confirm that within six months, tissue regeneration is nearly complete.
Key Takeaways
- Rapid tissue regeneration: The collagen gel transforms into a stable scaffold within minutes and facilitates full cartilage regrowth in about six months using the body’s healing mechanisms.
- Minimally invasive treatment: A single arthroscopic procedure replaces the need for open surgery, reducing infection risk, trauma, and recovery time significantly.
- Biodegradable solution: In contrast to permanent implants, this gel dissolves entirely post-regeneration, ensuring that only natural cartilage remains without lasting side effects.
- Regulatory approval and manufacturing: Both gel and liquid forms are CE-certified and manufactured under Good Manufacturing Practice, adhering to European Union standards for medical safety.
- Broad therapeutic potential: Beyond cartilage repair, the collagen gel shows great potential for treating a variety of musculoskeletal issues such as tendon injuries, bone reconstruction, and preserving joint function.
More details about this revolutionary development can be found directly from the Fraunhofer Institute’s official site, where ongoing research and applications are regularly updated.
German Scientists Create Injectable Gel That Regrows Cartilage in Minutes
German researchers at the Fraunhofer Institute for Interfacial Engineering and Biotechnology (IGB) have achieved a major breakthrough in regenerative medicine by developing an injectable gel that can regrow cartilage tissue within minutes. This innovative collagen-based implant offers a minimally invasive solution for cartilage repair that could transform how doctors treat joint injuries and degenerative conditions.
Revolutionary Gel Technology
The cell-free collagen gel represents a significant departure from traditional cartilage repair methods. Scientists inject the liquid gel arthroscopically directly into damaged cartilage areas, where it transforms into a stable scaffold within minutes of application. This biodegradable structure creates an optimal environment for natural healing processes to occur without requiring metal implants or synthetic materials.
The transformation process happens rapidly as the liquid collagen reorganizes into a three-dimensional framework that mimics natural cartilage structure. This science and technology advancement eliminates many complications associated with traditional surgical interventions while providing superior outcomes for patients.
Natural Regeneration Process
Once the scaffold forms, the gel facilitates a four-stage healing process that harnesses the body’s natural repair mechanisms:
- Gel injection through minimally invasive arthroscopic procedures
- Rapid scaffold formation creating a stable framework
- Stem cell and cartilage cell migration into the structure
- Complete tissue regeneration producing strong, natural cartilage
The biodegradable nature of the collagen gel means it gradually dissolves as new cartilage tissue develops, leaving behind only healthy, naturally regenerated cartilage. MRI scans from clinical studies show that cartilage defects in knee joints become almost completely filled within six months of treatment.
This breakthrough demonstrates how artificial intelligence and advanced research methods can accelerate medical discoveries. The Fraunhofer Institute’s approach focuses on creating conditions that allow the body’s own stem cells to perform the repair work, rather than introducing foreign cells or materials that might trigger immune responses.
The gel’s success lies in its ability to attract and support cellular migration from surrounding healthy tissue. Stem cells naturally present in joint fluid and nearby cartilage recognize the collagen scaffold as a suitable environment for growth and begin populating the structure immediately after injection.
https://www.youtube.com/watch?v=DNSN5fhuj3a
Revolutionary Results Show Complete Cartilage Repair in Six Months
MRI outcomes from recent clinical trials have shown remarkable success rates, with patients achieving near-complete cartilage regeneration within just six months of receiving the German-developed injectable gel treatment. These imaging results demonstrate that the gel creates a scaffold-like environment where natural cartilage cells can flourish and rebuild damaged tissue from within.
Advantages of the One-Step Minimally Invasive Approach
The revolutionary treatment offers several compelling benefits that set it apart from conventional surgical interventions:
- Requires only a single arthroscopic procedure instead of multiple surgical stages
- Eliminates the need for open surgery, reducing scarring and infection risks
- Provides faster patient recovery times compared to traditional cartilage repair methods
- Removes the psychological burden associated with permanent synthetic implants
- Significantly lowers long-term complication risks
I find the most striking aspect of this breakthrough lies in its temporary nature. Unlike permanent synthetic implants that remain in the body indefinitely, this injectable gel completely dissolves after facilitating cartilage regeneration. Patients receive only newly formed, natural cartilage tissue where damage once existed.
This non-permanent implant approach represents a paradigm shift in regenerative medicine. Traditional cartilage repair often requires harvesting healthy tissue from other body parts or installing artificial components that may wear down over time. The German gel eliminates these concerns by working with the body’s natural healing mechanisms rather than replacing them.
Recovery time improvements stand out as another significant advantage. Patients typically return to normal activities much sooner than those undergoing conventional cartilage surgery. The minimally invasive nature means smaller incisions, reduced tissue trauma, and less post-operative pain management requirements.
The psychological impact shouldn’t be underestimated either. Many patients struggle with knowing they carry permanent foreign materials in their bodies. This innovative gel treatment allows individuals to achieve complete healing without that mental burden, knowing their cartilage is entirely their own regenerated tissue.
Early adopters of this technology report enhanced quality of life and restored mobility that closely matches their pre-injury capabilities. The six-month timeframe also aligns well with typical rehabilitation schedules, allowing for proper tissue strengthening while regeneration occurs.
This advancement in science and technology opens doors for treating previously challenging cartilage damage cases. Athletes, elderly patients, and individuals with degenerative joint conditions now have access to a treatment option that was unimaginable just a few years ago. The success of this gel treatment may accelerate similar innovations in regenerative medicine, potentially revolutionizing how we approach tissue repair across various medical specialties.

Medical-Grade Manufacturing Meets European Safety Standards
The cartilage-regenerating gel emerges from a sophisticated manufacturing partnership between Fraunhofer IGB and German biotech company Amedrix GmbH, establishing new benchmarks for tissue engineering production. This collaboration operates under strict Good Manufacturing Practice (GMP) conditions, ensuring every batch meets pharmaceutical-grade quality standards that regulatory bodies demand for medical implants.
Advanced Collagen Processing and Cleanroom Standards
I observe that the manufacturing process begins with purified collagen extracted from animal tendons, then processed in controlled cleanroom environments that eliminate contamination risks. These cleanroom conditions maintain sterile atmospheres essential for producing medical devices that will interact directly with human tissue. The 215-square-meter production facility houses specialized equipment designed specifically for collagen purification and gel formulation, representing a significant investment in medical manufacturing infrastructure.
Amedrix GmbH brings proven expertise to this venture, having already secured EU market approval for previous collagen implants. Their track record provides confidence that this new liquid and gel format will navigate regulatory pathways successfully. The company’s experience with collagen-based medical devices translates into refined manufacturing protocols that ensure consistent product quality across production runs.
CE Certification and Regulatory Compliance
Both liquid and gel forms have earned CE certification since 2012 and 2013 respectively, marking them as compliant with European Union safety standards. This CE marking represents more than a regulatory checkbox – it demonstrates that the products meet strict biocompatibility requirements and performance standards that protect patient safety. I find that this certification process validates the manufacturing quality and clinical effectiveness that make the gel suitable for human use.
The regulatory approval protocols emphasize how GMP standards and clinical trials form the foundation for global deployment. These requirements aren’t bureaucratic hurdles but essential safeguards that ensure the gel performs consistently across different patient populations and medical settings. The collaboration between research institutions and commercial manufacturers creates a pathway where scientific innovation meets practical medical application.
Manufacturing under these stringent conditions costs more than standard production methods, but it guarantees that each dose of cartilage-regenerating gel meets the same exacting standards. This consistency becomes crucial when surgeons rely on predictable material properties during delicate joint procedures. The investment in proper manufacturing infrastructure pays dividends through improved patient outcomes and regulatory acceptance across international markets.

Vanishing Implants Could Transform Treatment Beyond Cartilage
The concept of biodegradable implants that disappear after guiding tissue regeneration represents a paradigm shift in medical treatment. Scientists are exploring how temporary structures can encourage natural healing while eliminating the long-term complications associated with permanent implants.
Expanding Applications Across Musculoskeletal Medicine
Researchers are applying the biodegradable scaffold approach to address a wide range of musculoskeletal conditions beyond cartilage repair. These innovative structures show promise for treating tendon injuries, where conventional surgical repairs often fail to restore full function. Bone reconstruction represents another frontier, particularly in cases where significant tissue loss occurs after trauma or cancer removal.
The potential applications extend to complex anatomical areas where traditional treatments fall short:
- Spinal reconstruction procedures that require temporary support during natural bone fusion
- Hip replacement surgeries where biodegradable components could reduce revision surgery needs
- Craniofacial reconstruction following traumatic injuries or congenital defects
- Joint preservation techniques that delay or eliminate the need for total joint replacement
Animal model trials currently underway demonstrate encouraging results across these diverse applications. Long-term safety assessments focus on ensuring complete biocompatibility and controlled degradation rates that match tissue regeneration timelines.
Redefining Patient Care Through Natural Healing
The advantages of vanishing implants over conventional permanent prosthetics become apparent when examining patient outcomes. Traditional implants carry risks of infection, loosening, and wear over time, often requiring revision surgeries. Biodegradable alternatives eliminate these long-term complications by gradually dissolving as the body’s own tissue takes over.
This regenerative approach could fundamentally alter how clinicians treat conditions like osteoarthritis. Instead of managing symptoms until joint replacement becomes necessary, doctors might intervene earlier with tissue engineering solutions that preserve natural joint function. The role of science in shaping technology continues to drive these medical breakthroughs.
Recovery timelines also improve significantly with biodegradable implants. Patients experience more natural healing processes without the foreign body response that permanent implants trigger. Physical therapy programs can be customized to work with the body’s regenerative capacity rather than around artificial components.
The economic implications extend beyond individual patient care. Reduced revision surgery rates and shorter recovery periods could decrease healthcare costs substantially. Healthcare systems worldwide are closely monitoring these developments as they seek sustainable solutions for aging populations with increasing musculoskeletal needs.
Current research focuses on optimizing degradation rates for different tissue types and anatomical locations. Scientists must balance providing adequate structural support during healing with ensuring complete dissolution at the appropriate time. Artificial intelligence advances help researchers model and predict optimal scaffold designs for specific patient conditions.
The manufacturing processes for these biodegradable implants require precise control over material properties and degradation characteristics. Advances in 3D printing technology enable custom-designed scaffolds that match individual patient anatomy and healing requirements. This personalized approach represents a significant departure from the one-size-fits-all methodology of traditional implants.
Regulatory pathways for biodegradable implants present unique challenges that differ from conventional medical devices. Safety profiles must account for both the active period when the implant provides structural support and the degradation phase when breakdown products enter the body. Clinical trials continue to generate data supporting the safety and efficacy of these innovative treatments.
The psychological benefits for patients cannot be overlooked. Many individuals express anxiety about having permanent foreign objects in their bodies. Vanishing implants address these concerns while providing superior functional outcomes. This combination of physical and emotional healing advantages positions biodegradable scaffolds as transformative technologies in regenerative medicine.

Next-Generation Smart Gels Combine Drug Delivery with Tissue Regeneration
The intersection of drug delivery and tissue engineering has reached a fascinating new frontier with dual-action smart hydrogels. These innovative materials don’t just promote tissue growth—they actively deliver therapeutic compounds while rebuilding damaged cartilage structures. I’ve been following these developments closely, and the implications for orthopedic medicine are extraordinary.
Revolutionary Dual-Drug Hydrogel Technology
Chinese researchers have developed nano-composite hydrogels that tackle osteoarthritis from multiple angles simultaneously. Their approach combines immediate inflammation control with long-term tissue regeneration, creating a comprehensive treatment strategy that goes far beyond traditional single-purpose interventions.
These sophisticated gels incorporate natural proteins alongside pharmaceutical compounds like dexamethasone and kartogenin. Dexamethasone provides rapid anti-inflammatory responses, while kartogenin supports the crucial process of chondrocyte differentiation—essentially helping cells transform into healthy cartilage tissue. The combination delivers both immediate relief and sustained healing, addressing the complex nature of degenerative joint conditions.
The structural design of these gels is equally impressive. With a porous architecture ranging from 10 to 30 micrometers, they achieve a remarkable gelation efficiency of 95%. This controlled porosity enables precise drug release patterns, with 80% of dexamethasone released alongside 40% of kartogenin over a 40-day period. Such controlled release mechanisms ensure therapeutic compounds remain active throughout the critical healing phases.
Clinical Applications and Treatment Advantages
These dual-functionality systems represent a significant shift toward personalized, minimally invasive orthopedic treatments. Unlike static implants that serve a single purpose, smart hydrogels adapt to the healing environment while continuously supporting regenerative processes. The ability to customize drug concentrations and release patterns means physicians can tailor treatments to individual patient needs and disease severity.
The applications extend beyond simple cartilage repair. I anticipate these technologies will revolutionize how we approach complex joint conditions, sports injuries, and age-related cartilage degeneration. The minimally invasive delivery methods reduce surgical risks while maximizing therapeutic outcomes—a combination that could transform patient care standards.
What makes this technology particularly exciting is its potential for integration with other emerging medical innovations. Artificial intelligence systems could optimize gel formulations based on patient-specific factors, while advances in scientific research continue pushing the boundaries of what’s possible in regenerative medicine.
The controlled release capabilities of these nano-composite hydrogels offer unprecedented precision in therapeutic delivery. Rather than flooding the treatment area with drugs that quickly metabolize and lose effectiveness, these systems maintain therapeutic concentrations for extended periods. This sustained approach reduces the need for repeated interventions while ensuring optimal healing conditions persist throughout the recovery process.
Looking ahead, I expect these dual-drug hydrogel technologies will become standard treatment options for various musculoskeletal conditions. Their ability to combine immediate symptom relief with long-term tissue restoration addresses one of medicine’s greatest challenges—providing both rapid patient comfort and lasting therapeutic benefits. The versatility of these systems suggests applications will expand beyond cartilage repair into other tissue engineering applications, potentially revolutionizing how we approach complex medical conditions requiring both pharmaceutical intervention and structural repair.

How This Technology Changes the Future of Joint Surgery
The groundbreaking German gel technology represents a revolutionary departure from conventional surgical approaches that have dominated orthopedic medicine for decades. Rather than relying on permanent metal or plastic implants that require eventual replacement, this innovative gel works with the body’s natural healing mechanisms to regenerate actual cartilage tissue. I see this as a fundamental shift that transforms joint surgery from a mechanical repair process into a biological restoration procedure.
Moving Beyond Permanent Implants
Traditional joint replacements present significant long-term challenges, particularly for younger patients who face the prospect of multiple revision surgeries throughout their lifetimes. The German gel technology offers a compelling alternative by providing temporary scaffolding that supports the body’s own regenerative processes. Once the cartilage regrows, the gel naturally dissolves, leaving behind healthy, functional tissue that integrates seamlessly with existing joint structures.
This biological approach proves especially valuable in complex reconstructive cases where conventional implants carry heightened surgical risks. Procedures involving unusual bone geometries or previous surgical complications often benefit from solutions that adapt to the body’s unique anatomy rather than forcing mechanical components into challenging spaces. Science and technology continue to evolve in ways that support these more sophisticated treatment approaches.
Transforming Treatment for Younger Patients
The technology holds particular promise for younger patients who suffer joint damage from sports injuries, genetic conditions, or trauma. These individuals previously faced limited options that often included:
- Living with pain and reduced mobility for years before qualifying for joint replacement
- Accepting permanent implants that would require multiple revisions over their lifetime
- Undergoing extensive rehabilitation with uncertain outcomes
- Dealing with activity restrictions that significantly impact quality of life
The regenerative gel opens new possibilities by potentially restoring natural joint function without the long-term complications associated with artificial implants. This advancement aligns with broader trends in artificial intelligence and medical innovation that prioritize biological solutions over purely mechanical interventions.
The integration of this gel technology with stem cell therapies and personalized medicine approaches creates even more exciting prospects. Doctors could potentially combine the patient’s own stem cells with the regenerative gel to create highly customized treatment protocols that address individual healing patterns and genetic factors. This personalized approach represents a significant evolution from the one-size-fits-all mentality that has characterized traditional joint surgery.
Future applications might include treating previously irreversible cartilage damage in areas where surgery was considered too risky or ineffective. The gel’s ability to promote natural healing could make intervention possible in joints that were previously considered beyond repair. Additionally, the technology might serve as a preventive measure, addressing early-stage cartilage damage before it progresses to the point where major reconstructive surgery becomes necessary.
The broader implications extend beyond individual patient outcomes to reshape surgical training and medical education. Orthopedic surgeons will need to develop new skills that bridge traditional surgical techniques with regenerative medicine principles. This evolution reflects the same kind of technological advancement seen in other fields where physics and scientific innovation drive practical applications.
Recovery protocols will also evolve significantly as patients experience healing processes that more closely mirror natural biological repair rather than adaptation to foreign implants. Physical therapy approaches may need adjustment to support cartilage regeneration rather than simply teaching patients to accommodate mechanical replacements.
The technology positions joint surgery to become increasingly precise and individualized, moving away from standardized procedures toward treatments that respond to each patient’s specific biological profile and healing capacity. This represents not just an incremental improvement in surgical technique, but a fundamental reimagining of how medicine approaches joint repair and restoration.
Sources:
ScienceDaily – Collagen for the knee: Gel-like implant invented (Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB)
News-Medical.net – Breakthrough dual-drug hydrogel promotes cartilage repair in osteoarthritis
Snopes – German scientists developed gel to help heal damaged joint cartilage?
The Vanishing Healer blog – Synthetic Cartilage That Regenerates Then Disappears (Michele Gargiulo, inspired by Fraunhofer IGB)
Instagram post – Injected Collagen Gel Regrows Knee Cartilage Naturally! Germany’s…