ChondroFiller represents a breakthrough in cartilage repair technology, offering a single-procedure solution that uses a cell-free collagen hydrogel to treat focal cartilage defects measuring up to 3 cm².
Unlike traditional multi-stage treatments that involve cell harvesting, cultivation, and multiple surgical interventions, ChondroFiller solidifies within just 3–5 minutes during arthroscopic surgery. This rapid-setting technology forms an immediate scaffold that encourages natural cartilage regeneration, offering a less invasive and more efficient solution for patients suffering from localized cartilage damage.
Key Takeaways
- Single-procedure treatment: Eliminates the need for multiple surgeries, cell harvesting, or bone microfracturing, significantly simplifying the treatment process compared to traditional cartilage repair methods.
- Targeted application: Most effective for grades III–IV cartilage defects up to 3 cm² in younger, active adults with focal damage rather than widespread joint degeneration.
- Natural regeneration support: Creates a 3D collagen scaffold that promotes the body’s own chondrocytes and stem cells to migrate and form new hyaline-like cartilage containing type II collagen.
- Proven clinical results: Over 20,000 worldwide applications since 2013 demonstrate that more than 80% of appropriate candidates achieve good to excellent long-term outcomes across multiple joint types.
- Advanced biomaterial design: Manufactured in Germany under strict European standards, the cell-free hydrogel eliminates immunological rejection risks while providing immediate stabilization that allows for earlier patient mobilization.
To learn more about this technology, visit the official ChondroFiller website.
A Revolutionary Single-Procedure Solution for Cartilage Repair
ChondroFiller represents a significant advancement in cartilage repair technology, offering patients a streamlined approach that eliminates the need for multiple surgical procedures. This collagen-based, cell-free hydrogel transforms cartilage treatment by requiring only one minimally invasive arthroscopic surgery, dramatically simplifying what was once a complex treatment process.
The procedure begins with precise diagnostic imaging through MRI or direct arthroscopy to confirm the defect’s size and severity. Surgeons focus specifically on grades III–IV cartilage defects measuring 3 cm² or smaller, ensuring optimal treatment outcomes. This diagnostic precision allows for targeted intervention that maximizes the gel’s effectiveness while minimizing unnecessary tissue disruption.
During the arthroscopic procedure, surgeons first clean damaged tissue from the defective cartilage area, preparing a clean foundation for the hydrogel application. ChondroFiller’s unique formulation adapts seamlessly to both the size and shape of each individual lesion, creating a customized fit that traditional treatments often struggle to achieve. The gel’s remarkable property of solidifying within 3–5 minutes creates a stable matrix that immediately begins supporting the healing process.
Simplified Treatment Without Complex Preparations
Unlike conventional cartilage repair methods, ChondroFiller eliminates several complicated steps that traditionally burden both patients and surgeons. The procedure requires no bone microfracturing, avoiding the additional trauma and recovery time associated with this invasive technique. Similarly, the treatment doesn’t rely on fibrin glue, removing another variable that can complicate the healing process.
Perhaps most significantly, ChondroFiller completely bypasses the need for cell harvesting or cultivation. Traditional cell-based therapies often require patients to undergo initial procedures to extract healthy cartilage cells, followed by weeks of laboratory cultivation before reimplantation. This extended timeline not only delays treatment but also introduces risks of contamination or cell viability issues. The cell-free nature of ChondroFiller means surgeons can proceed directly to treatment, much like how robots adapt their form to changing environments.
The collagen gel application creates an immediate scaffold that supports natural cartilage regeneration without relying on externally cultivated cells. This scaffold provides the structural framework necessary for the body’s own repair mechanisms to take over, encouraging healthy tissue growth from the surrounding cartilage.
Recovery advantages become apparent almost immediately following the procedure. Patients typically experience faster healing times compared to traditional multi-stage treatments, with reduced overall pain levels throughout the recovery period. The minimally invasive nature of the arthroscopic approach results in significantly less scarring, both visible and internal, which contributes to improved joint mobility and reduced long-term complications.
MRI guidance throughout the procedure ensures precise placement and optimal coverage of the affected area. This imaging support allows surgeons to verify complete lesion coverage while avoiding healthy tissue, maximizing treatment effectiveness while minimizing collateral damage.
The single-procedure approach also reduces the psychological burden on patients who would otherwise face multiple surgeries spread over months. Financial benefits accumulate as well, since patients avoid the costs associated with multiple procedures, extended rehabilitation periods, and repeated facility fees.
ChondroFiller’s immediate stabilization properties mean patients can begin gentle mobilization sooner than with traditional treatments. The stable matrix created by the hardened gel provides confidence for early movement protocols, potentially preventing the joint stiffness that often complicates longer immobilization periods.
This revolutionary approach transforms cartilage repair from a lengthy, multi-stage process into a single, definitive treatment. The combination of advanced biomaterial science with proven arthroscopic techniques creates a solution that addresses both the technical challenges of cartilage repair and the practical concerns of patients seeking effective, efficient treatment options.
How ChondroFiller Transforms Damaged Cartilage Into New Tissue
ChondroFiller functions as a sophisticated three-dimensional scaffold that facilitates the body’s natural healing processes. I’ve observed how this innovative gel creates an optimal environment for the body’s own chondrocytes and stem cells to migrate into damaged areas, where they can proliferate and begin synthesizing new cartilage tissue.
The Biological Mechanism of Cartilage Regeneration
The gel establishes a supportive 3D matrix that actively promotes chondrocyte migration throughout the treatment area. Once these cells settle into the scaffold, they begin their essential work of proliferation and regeneration. I find it particularly fascinating how the process results in the formation of hyaline-like cartilage containing type II collagen, which is synthesized entirely by the patient’s own host cells. This biological approach ensures that the new tissue integrates seamlessly with existing cartilage structures.
The transformation process relies on the body’s inherent capacity for self-repair, with ChondroFiller simply providing the structural framework needed for optimal healing. Unlike synthetic alternatives, this method harnesses natural cellular processes to achieve lasting cartilage restoration.
Clinical Evidence and Long-Term Outcomes
Clinical research demonstrates impressive results across various patient populations. In a prospective hip study involving 26 adults with lesions greater than 2 cm², 17 out of 21 patients achieved good to excellent results after 3-5 years of follow-up. I note that these outcomes were measured using both MRI imaging and functional scoring systems, providing comprehensive assessment data.
The broader clinical experience spans over 20,000 patients since 2013, consistently showing favorable safety and effectiveness profiles for focal cartilage repair. These extensive real-world applications provide substantial evidence of the treatment’s reliability and predictable outcomes.
Longitudinal study results reveal that more than 80% of appropriate candidates experience good to excellent long-term results. This success rate reflects careful patient selection combined with the biological effectiveness of the scaffold approach. I consider these outcomes particularly significant because they represent sustained improvement over extended periods, indicating genuine tissue regeneration rather than temporary symptom relief.
The MRI outcomes consistently show structural improvements in cartilage architecture, while functional scores demonstrate meaningful improvements in patient mobility and pain reduction. These dual measures confirm that ChondroFiller doesn’t just mask symptoms but facilitates actual tissue healing that patients can feel in their daily activities.
Proven Clinical Results Across Multiple Joint Types
ChondroFiller has established itself as a versatile solution for treating small, well-defined cartilage defects across multiple joint locations. I’ve observed its application in knees, hips, shoulders, ankles, and select small hand joints, demonstrating consistent results when applied to appropriate candidates. The treatment targets patients with cartilage damage measuring up to 3 cm², making it particularly effective for focal defects rather than widespread joint deterioration.
Clinical Evidence and Documentation
Clinical studies provide compelling evidence of ChondroFiller’s effectiveness through MRI-documented cartilage restoration. These studies specifically focus on Grade III/IV lesion treatment measuring ≤3 cm², showing measurable improvement in cartilage structure and function. The MRI documentation serves as objective proof of tissue regeneration, allowing physicians to track progress and validate treatment success over time.
The technology has achieved remarkable results in younger, active adults experiencing early cartilage loss. Unlike treatments designed for elderly patients or those with advanced osteoarthritis, ChondroFiller performs optimally in individuals whose joints retain overall structural integrity. This selectivity ensures that patients receive treatment when their bodies can best respond to cartilage regeneration therapy.
Global Safety Profile and Applications
Since 2013, over 20,000 clinical uses worldwide have established ChondroFiller’s safety profile and effectiveness. This extensive clinical experience spans diverse patient populations and joint applications, providing physicians with confidence in treatment outcomes. The technology’s track record demonstrates consistent performance across different healthcare systems and patient demographics.
Healthcare providers must understand that ChondroFiller isn’t designed for advanced osteoarthritis cases where joint-wide cartilage loss has occurred. Instead, it excels in treating isolated cartilage defects where surrounding tissue remains healthy. This specificity allows for targeted intervention that can prevent larger cartilage problems from developing while maintaining joint function in active individuals.
The treatment’s versatility across multiple joint types makes it valuable for athletes and active individuals who depend on joint mobility for their lifestyle or profession. Much like how innovative technology debuts change transportation possibilities, ChondroFiller represents a significant advancement in cartilage restoration options for specific patient populations.
Joint-specific applications vary based on anatomical considerations and patient activity levels:
- Knee: Often involved in high-impact athletes.
- Shoulder: Frequently used for overhead motion athletes or workers.
- Ankle: Typically affected by traumatic injuries.
- Hip: Focuses on young adults with focal cartilage damage before arthritis develops.
Patient selection criteria remain critical for optimal outcomes. Candidates should have focal cartilage defects without significant joint space narrowing or bone-on-bone contact. Age considerations favor younger patients whose healing capacity supports cartilage regeneration, though chronological age alone doesn’t determine candidacy.
The treatment protocol involves precise application techniques that ensure optimal cell distribution and adherence to defect sites. Proper patient preparation and post-treatment care protocols contribute significantly to successful outcomes, requiring coordination between surgical teams and rehabilitation specialists.
Long-term follow-up data continues to support ChondroFiller’s effectiveness in maintaining cartilage integrity over extended periods. Patients who receive treatment within appropriate indications show sustained improvement in joint function and pain reduction, validating the technology’s role in preventing progression to more severe joint conditions.
Healthcare providers considering ChondroFiller should evaluate each patient’s specific defect characteristics, activity goals, and healing potential. The treatment’s proven track record across multiple joint types offers confidence for addressing focal cartilage defects in appropriately selected candidates, providing a viable alternative to more invasive surgical procedures or long-term symptom management approaches.
What Makes ChondroFiller Different From Traditional Treatments
ChondroFiller Liquid represents a significant departure from conventional cartilage treatment approaches. While traditional methods often focus on symptom management or invasive procedures, this innovative solution takes a fundamentally different path by utilizing Type I collagen derived from veterinary-monitored rat tail tendons. The manufacturing process adheres to strict German and European standards, ensuring consistent quality and safety for patients seeking cartilage regeneration alternatives.
Advanced Biologic Composition Without Cellular Complications
The collagen scaffold technology sets ChondroFiller apart from both traditional treatments and newer cellular therapies. Unlike stem cell procedures or cell transplantation methods, this cell-free hydrogel eliminates the complexities associated with cell culturing and donor site complications. Patients don’t face the risks of immunological rejection that often accompany cellular therapies, since the acellular composition removes this concern entirely.
Traditional treatments typically require extensive procedures such as microfracturing or joint replacement surgery. ChondroFiller’s biocompatible matrix offers a less invasive alternative that doesn’t demand additional donor site morbidity. The regenerative therapy approach focuses on creating an environment where natural healing can occur, rather than simply masking pain or temporarily addressing symptoms.
Cost-Effective Regenerative Approach
Economic considerations make ChondroFiller an attractive option compared to complex cell-based therapies. The reduced procedure complexity translates to lower overall costs while maintaining effectiveness in cartilage regeneration. Traditional pain management approaches often require ongoing treatments and medications, creating long-term financial burdens for patients.
The absorbability of the collagen matrix means the body naturally processes the material as regeneration occurs. This characteristic eliminates concerns about permanent foreign materials remaining in the joint. Instead of requiring multiple procedures or extended recovery periods like traditional surgical interventions, ChondroFiller supports the body’s natural regenerative processes.
Biocompatibility remains a cornerstone of this treatment’s effectiveness. The carefully selected collagen source and manufacturing standards ensure that patients receive a product designed specifically for optimal integration with existing tissue. Rather than fighting against the body’s natural processes, this regenerative therapy works in harmony with existing biological mechanisms.
The approach differs markedly from traditional treatments that often view cartilage damage as a permanent condition requiring ongoing management. ChondroFiller’s philosophy centers on actual regeneration rather than simple symptom control. This paradigm shift offers hope for patients who previously faced limited options beyond pain management or major surgical interventions.
Quality control measures during manufacturing ensure consistent results across treatments. The German and European standards provide rigorous oversight that traditional treatments may lack. This attention to detail creates confidence in both medical professionals and patients considering this innovative approach to cartilage restoration.
Clinical applications demonstrate how this cell-free matrix supports natural healing processes without the complications associated with cellular therapies. The technology bridges the gap between conservative treatment options and major surgical procedures, offering an intermediate solution that addresses root causes rather than just symptoms. Advanced materials science continues to drive innovations in medical treatments like ChondroFiller.
Understanding the Right Candidates and Limitations
Chondrofiller demonstrates optimal effectiveness in patients presenting with early-stage cartilage damage, specifically grades III-IV lesions measuring up to 3 cm² in size. I’ve observed that proper patient selection directly influences treatment success, making this criterion essential for practitioners considering this intervention.
Ideal Candidate Profile
The most successful outcomes occur in younger, active adults who present with focal cartilage defects rather than widespread joint degeneration. These patients typically maintain healthy surrounding cartilage tissue and demonstrate good overall joint mechanics. The procedure works best when treating isolated damage areas, as opposed to addressing systemic joint deterioration.
Several key factors define appropriate candidates:
- Cartilage lesions measuring 3 cm² or smaller in total area
- Grade III-IV cartilage damage with intact subchondral bone
- Active lifestyle with motivation for rehabilitation compliance
- Absence of significant joint malalignment or instability
- Realistic expectations regarding recovery timeline and outcomes
Advanced osteoarthritis patients don’t achieve comparable results with chondrofiller treatment. The procedure shows significantly reduced effectiveness in individuals with Tönnis grade 2-3 osteoarthritis, where extensive joint space narrowing and bone changes limit healing potential. Large or unstable lesions exceeding 3 cm² also present challenges that compromise treatment success.
I find that patient age plays a crucial role in determining candidacy, though chronological age alone shouldn’t exclude treatment consideration. Biological age, activity level, and overall joint health often provide better indicators of potential success than numerical age alone. However, like rare natural phenomena that require specific conditions to occur, chondrofiller requires precise circumstances for optimal results.
Current research continues to establish long-term efficacy through large, randomized trials, meaning practitioners must carefully weigh available evidence against individual patient circumstances. While early studies show promising results, the absence of extensive long-term data requires honest discussions about treatment expectations and potential alternatives.
Critical limitations include the procedure’s inability to address widespread cartilage loss, significant joint deformity, or inflammatory conditions affecting the entire joint space. Patients with unrealistic expectations about immediate return to high-impact activities may experience disappointment, as recovery typically requires months of dedicated rehabilitation.
The selection process demands thorough evaluation of cartilage defect characteristics, surrounding tissue health, patient compliance potential, and alternative treatment options. I recommend comprehensive imaging assessment combined with detailed patient history to ensure appropriate candidate identification before proceeding with chondrofiller treatment.
ChondroFiller Product Specifications and Availability
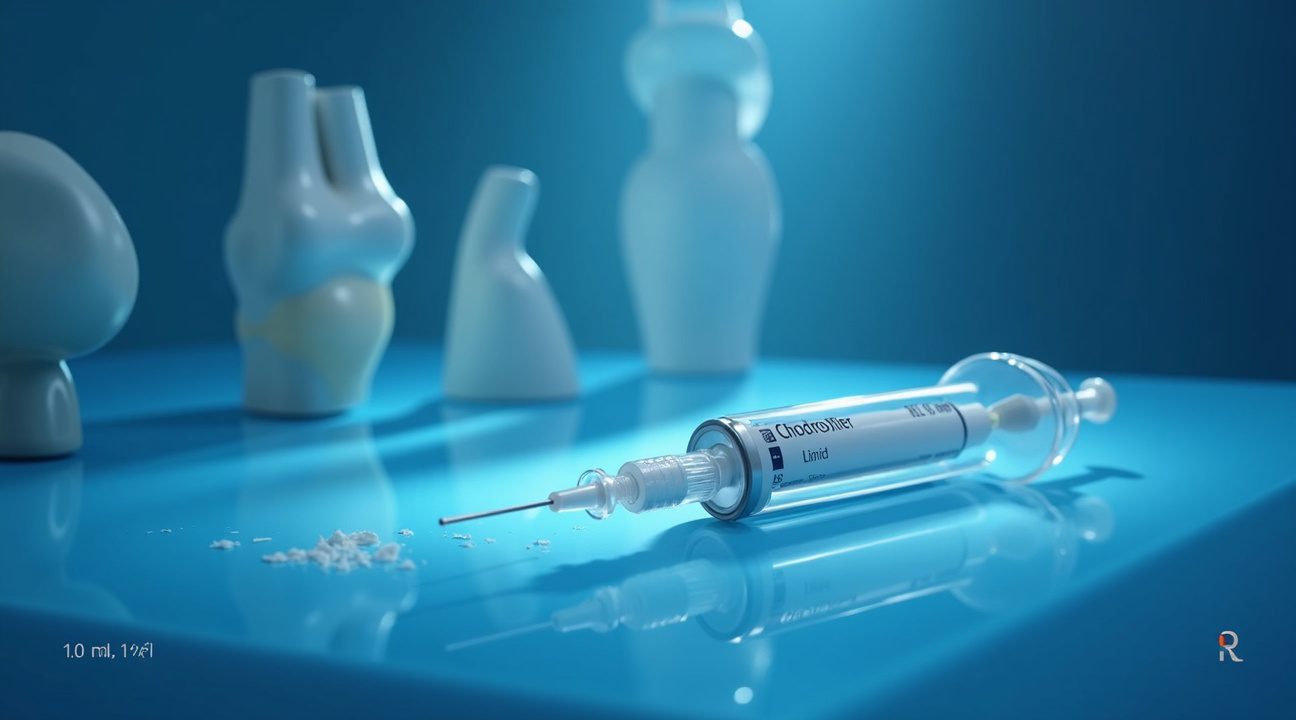
ChondroFiller Liquid arrives in an innovative two-chamber syringe system that ensures optimal performance through immediate mixing and application. This design maintains the integrity of the biological components until the precise moment of use, eliminating concerns about premature degradation or contamination. The dual-chamber configuration allows medical professionals to combine the necessary components at the point of care, providing fresh, active material for each procedure.
Available Volumes and Sizing Options
The product comes in three distinct volume options to accommodate varying defect sizes and surgical requirements. Healthcare providers can select from 1.0 ml, 1.5 ml, and 2.3 ml presentations, ensuring precise volume matching for different cartilage lesions. This range allows surgeons to optimize material usage while minimizing waste, particularly important given the specialized nature of the product.
Smaller volumes work well for focal defects in joints like the ankle or hand, while larger volumes accommodate more extensive damage in major weight-bearing joints.
Manufacturing Standards and Global Approval
Meidrix biomedicals GmbH produces ChondroFiller in Germany through a collaboration with Fraunhofer IGB, maintaining strict quality controls throughout the manufacturing process. The product holds CE certification, meeting European medical device standards for safety and efficacy. These rigorous manufacturing protocols ensure consistent product quality and performance across all batches.
Since receiving approval, ChondroFiller has accumulated over 20,000 clinical uses worldwide since 2013, establishing a substantial track record of real-world application. This extensive clinical experience spans multiple countries and healthcare systems, providing valuable data on long-term outcomes and safety profiles.
ChondroFiller receives approval for treating cartilage damage classified as grades III and IV, representing significant cartilage loss that requires intervention. The product’s indications cover major joints including the knee, hip, shoulder, and ankle, as well as smaller joints in the hand. This broad approval range reflects the versatility of the biomaterial and its ability to function effectively across different joint environments and mechanical loads.
Surgeons can apply ChondroFiller in various anatomical locations, from high-load bearing areas like the knee to more complex joints like the shoulder that require different mechanical properties.
The German manufacturing base provides several advantages for quality assurance and regulatory compliance. Germany’s pharmaceutical and medical device industries maintain some of the world’s highest standards, with comprehensive oversight and validation processes. The collaboration with Fraunhofer IGB, a renowned research institute, brings additional scientific rigor to the development and production process. This partnership combines commercial manufacturing expertise with cutting-edge research capabilities, ensuring the product reflects the latest advances in cartilage repair technology.
Clinical adoption has grown steadily since the initial launch, with usage spanning orthopedic practices, specialized sports medicine centers, and major hospital systems. The accumulation of over 20,000 clinical applications provides substantial real-world evidence for both safety and effectiveness. This experience base includes:
- Diverse patient populations
- Varying defect sizes
- Different surgical approaches
These factors contribute to a comprehensive understanding of optimal application techniques.
The CE marking process requires extensive documentation of safety and performance data, including:
- Biocompatibility testing
- Mechanical property analysis
- Clinical evaluation
ChondroFiller successfully completed these requirements, demonstrating its suitability for human use in cartilage repair applications. The certification also enables distribution throughout the European Union and other regions that recognize CE marking, expanding access for patients and healthcare providers globally.
Sources:
London Cartilage: “ChondroFiller: A Breakthrough in Cartilage Repair”
Germanten Hospitals: “Best Chondrofiller Injections for Joint Health”
Meidrix Biomedicals GmbH: “ChondroFiller”
IMAB Journal: “Implantation of ChondroFiller Liquid as a scaffold material for the regeneration of articular cartilage”
NCBI (PMC): “Arthroscopic utilization of ChondroFiller gel for the treatment of hip cartilage lesions”
IOMC World: “The Use of an Acellular Collagen Matrix ChondroFiller Liquid for Trapeziometacarpal Osteoarthritis”
Patient Choice International: “Chondrofiller”