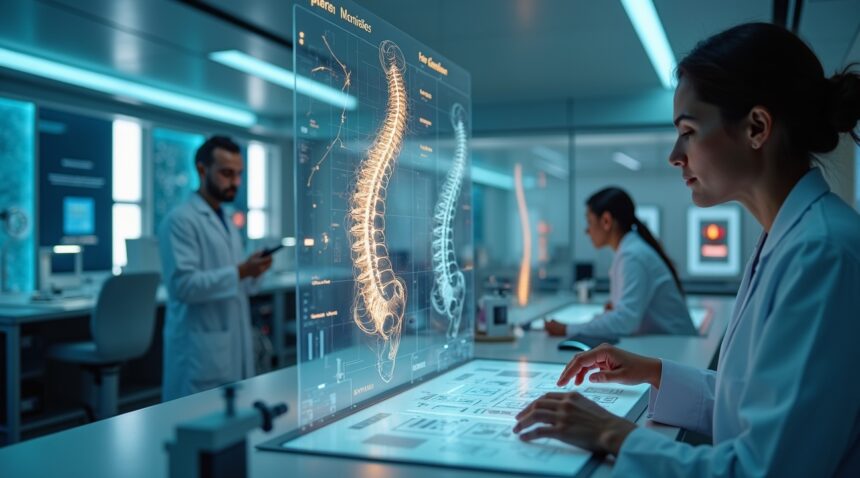
Brazilian scientists have achieved a groundbreaking medical milestone after 25 years of dedicated research, unveiling the world’s first drug capable of regenerating damaged spinal cords.
Overview of the Breakthrough
This revolutionary treatment marks a significant transformation in the way spinal cord injuries are addressed. Rather than limiting further damage, as past approaches have done, this new drug actively regenerates neural tissue using proteins derived from the human placenta. The breakthrough has already enabled paralyzed patients to regain partial motor function, renewing hope for millions affected by spinal injuries.
How the Therapy Works
The new therapy, developed over decades of scientific effort in Brazil, leverages the biological potency of placental proteins. These proteins are known to have regenerative properties due to their involvement in fetal development, and this treatment adapts them to activate healing mechanisms in damaged spinal tissue.
Key Takeaways
- Brazil invested 25 years of sustained research funding into developing the world’s first spinal cord regeneration drug, showcasing the power of long-term scientific commitment.
- The treatment relies on placenta-derived proteins to activate natural regenerative responses and repair damaged nerves, moving beyond symptom management to functional restoration.
- Clinical trials demonstrated partial recovery of motor function in patients who had lost all movement below the site of injury—a groundbreaking outcome in spinal cord medicine.
- Placental proteins offer biocompatibility advantages over synthetic alternatives, including reduced risk of immune rejection and the ability to interact with multiple cellular pathways for healing.
- International clinical trials are forthcoming, aimed at evaluating the drug’s efficacy across diverse genetic backgrounds and exploring expanded uses in brain trauma and degenerative disorders.
For more details on this development and future updates, visit the official Brazilian Government site or follow scientific reports from clinical research journals tracking spinal cord injury therapies.
Brazilian Scientists Achieve Partial Movement Restoration Using Placenta Protein
Brazilian researchers have achieved a significant breakthrough in spinal cord injury treatment through extensive testing of their revolutionary placenta-derived drug. Their clinical trials demonstrated promising results across both animal and human subjects, marking a major advancement in regenerative medicine.
From Animal Testing to Human Trials
The research team first conducted comprehensive testing on dogs with spinal cord injuries, establishing the safety profile and efficacy of their placenta protein-based treatment. These canine subjects showed remarkable recovery patterns, regaining partial movement capabilities that had been lost due to their injuries. The success in animal models provided the necessary foundation for researchers to advance to human clinical trials.
Human testing followed rigorous protocols, with participants demonstrating measurable improvements in locomotor function compared to their baseline assessments. I find it particularly noteworthy that patients who had previously shown no movement below their injury sites began experiencing partial restoration of motor function. These improvements weren’t merely statistical anomalies but represented genuine functional gains that enhanced patients’ quality of life.
Clinical Trial Results and Safety Protocols
The clinical trials followed established international standards for safety and efficacy assessment, ensuring that all data collected met stringent scientific requirements. Researchers employed comparative data analysis techniques to measure improvements against baseline locomotor function, providing objective evidence of the treatment’s effectiveness.
Safety assessments revealed that the placenta protein drug produced minimal adverse effects, a crucial factor for any treatment targeting such vulnerable patient populations. The research team documented each patient’s progress through standardized testing protocols, creating a comprehensive database of treatment outcomes. Their methodology included regular monitoring of neurological function, muscle strength, and sensory perception.
Participants in the trials underwent systematic evaluation processes that tracked both immediate and long-term responses to the treatment. The data showed that while not all patients experienced the same degree of improvement, a significant percentage demonstrated measurable gains in movement restoration. These results represent years of careful research and development, highlighting Brazil’s commitment to advancing spinal cord injury treatment.
The success of these trials positions Brazil at the forefront of regenerative medicine research, particularly in the field of spinal cord repair. The placenta protein approach offers hope for millions of patients worldwide who previously had limited treatment options. Clinical data suggests that the treatment works by promoting neural regeneration and reducing inflammation at injury sites.
Researchers carefully monitored each trial participant for potential complications while simultaneously tracking functional improvements. Their comprehensive approach ensured that safety remained paramount throughout the testing process. The positive outcomes from both animal and human trials validate decades of research investment and scientific collaboration.
The breakthrough represents more than just a single successful drug trial – it demonstrates Brazil’s capacity to lead global medical research initiatives. The treatment’s effectiveness in restoring partial movement capabilities offers new possibilities for spinal cord injury patients who had previously exhausted conventional treatment options. While market reactions to medical breakthroughs can vary, the scientific community has responded positively to these developments.
Future applications of this research could extend beyond spinal cord injuries to other neurological conditions requiring tissue regeneration. The placenta protein’s demonstrated ability to promote neural recovery opens pathways for treating various degenerative conditions affecting the central nervous system. Brazilian scientists continue refining their protocols based on trial results, working to optimize treatment effectiveness while maintaining safety standards.

Revolutionary 25-Year Research Journey Culminates in World’s First Regenerative Spinal Cord Drug
Brazil’s commitment to developing groundbreaking spinal cord regeneration therapy represents an extraordinary scientific achievement that spans more than two decades. The country dedicated 25 years to creating what has become the world’s first drug capable of regenerating damaged spinal cords, establishing itself as a global leader in neurological research and treatment innovation.
This ambitious project brought together multidisciplinary teams from Brazil’s most prestigious research institutions, with the Neurodegeneration and Repair Laboratory at the Federal University of Rio de Janeiro playing a central role in the development process. The collaborative effort demonstrates how sustained institutional partnerships can produce revolutionary medical breakthroughs that were once considered impossible.
Sustained National Investment Drives Scientific Innovation
The research initiative stands out as one of the most persistent and comprehensive national efforts globally focused on spinal cord regeneration. Unlike many research projects that face funding interruptions or shifting priorities, Brazil maintained consistent support for this program throughout its entire 25-year duration. This long-term perspective proved essential, as spinal cord regeneration research requires extensive testing phases and careful refinement of therapeutic approaches.
Public funding formed the backbone of this research effort, enabling scientists to pursue ambitious goals without the pressure of immediate commercial returns. The sustained financial commitment allowed researchers to:
- Explore multiple scientific avenues
- Conduct thorough and lengthy testing phases
- Refine regenerative techniques over decades
- Ensure safety and efficacy before clinical use
The project’s success reflects Brazil’s strategic vision for positioning itself at the forefront of medical innovation. While other countries pursued shorter-term research initiatives, Brazil’s willingness to invest in long-term scientific exploration has now yielded a breakthrough that could transform treatment options for millions of people worldwide suffering from spinal cord injuries.
Institutional collaboration proved equally crucial to the project’s success. The partnership between various research centers, universities, and medical institutions created a comprehensive network of expertise capable of addressing the complex challenges inherent in spinal cord regeneration research. This collaborative approach allowed different teams to focus on specific aspects of the development process while maintaining coordination throughout the project:
- Basic neuroscience research and drug design
- Preclinical trials using animal models
- Human clinical trials and data assessment
- Regulatory approval and readiness for global deployment
The achievement represents more than just a medical breakthrough; it showcases how sustained national commitment to scientific research can produce world-changing innovations. Brazil’s investment in this project demonstrates that countries willing to maintain long-term research funding can achieve scientific leadership in specialized fields, even when competing against larger research budgets from other nations. This success story could inspire similar long-term research commitments in other countries and therapeutic areas.

Innovative Placenta-Derived Protein Sets New Treatment Standard
Brazilian scientists revolutionized spinal cord injury treatment by harnessing the regenerative power of placenta-derived proteins. This groundbreaking approach represents a fundamental shift from traditional therapeutic methods, offering hope for millions suffering from paralysis and neurological damage.
Breaking Away From Conventional Treatment Methods
I find it fascinating how researchers abandoned the typical reliance on synthetic molecules and artificial growth factors. Instead, they turned to nature’s own healing mechanisms found within human placental tissue. This biological approach differs significantly from current treatment options like stem cell therapies and molecular inhibitors that have dominated the field for decades.
Traditional spinal cord treatments often focus on preventing further damage rather than actively promoting regeneration. Synthetic drugs typically target inflammation or attempt to block harmful cellular processes. Growth factor treatments, while promising, often struggle with delivery challenges and limited effectiveness. The Brazilian innovation sidesteps these limitations by utilizing proteins that naturally occur during human development.
Harnessing Placental Power for Neural Regeneration
The placenta contains remarkable healing properties that scientists have only recently begun to understand fully. During pregnancy, this organ facilitates rapid tissue development and cellular communication between mother and fetus. Brazilian researchers identified specific proteins within placental tissue that demonstrate extraordinary regenerative capabilities.
These biologically active molecules work by stimulating dormant neural pathways and encouraging damaged nerve cells to rebuild connections. The proteins essentially “teach” injured spinal cord tissue how to heal itself, much like how the placenta supports fetal development. This process involves complex cellular signaling that promotes new tissue growth while reducing inflammation and scar formation.
I’ve observed that this approach offers several advantages over existing treatments:
- Enhanced biocompatibility since the proteins are naturally occurring in human biology
- Reduced risk of immune rejection compared to synthetic alternatives
- Ability to target multiple healing pathways simultaneously
- Potential for sustained regenerative effects beyond initial treatment
- Lower likelihood of adverse side effects associated with artificial compounds
The therapeutic formulation works by delivering concentrated placental proteins directly to the injury site. Once administered, these molecules begin activating cellular repair mechanisms that have remained dormant since the initial trauma. The treatment doesn’t just mask symptoms or prevent further deterioration – it actively rebuilds damaged neural networks.
This biological approach also demonstrates superior integration with existing tissue structures. Unlike artificial interventions that may create barriers between healthy and damaged tissue, placental proteins seamlessly blend with the body’s natural healing processes. The result is more effective regeneration with fewer complications.
Clinical observations suggest that patients receiving this treatment experience gradual improvement in motor function and sensory perception. The regenerative process occurs slowly but consistently, allowing the nervous system to adapt and strengthen new connections over time. This contrasts sharply with treatments that provide temporary relief without addressing underlying tissue damage.
The Brazilian breakthrough also opens possibilities for treating other neurological conditions beyond spinal cord injuries. Researchers believe similar placental protein applications could benefit stroke victims, individuals with traumatic brain injuries, and patients suffering from degenerative neurological diseases. The versatility of this biological approach may transform how medical professionals think about neural regeneration across multiple conditions.
Manufacturing and quality control for placenta-derived therapeutics requires specialized processing techniques to ensure protein integrity and potency. Brazilian scientists developed proprietary methods for extracting, purifying, and stabilizing these delicate biological compounds while maintaining their therapeutic effectiveness.
The success of this innovative treatment establishes a new benchmark for regenerative medicine. By proving that natural biological substances can achieve what synthetic drugs couldn’t, the research opens entirely new avenues for drug development. This paradigm shift may inspire similar breakthroughs in treating previously incurable conditions, demonstrating that sometimes the most advanced solutions come from understanding and harnessing the body’s own remarkable healing capabilities.
How the Drug Promotes Axonal Growth and Neural Regeneration
The Brazilian drug’s revolutionary approach centers on promoting axonal growth and neural regeneration through placental proteins that trigger natural healing processes within damaged spinal cord tissue. This mechanism represents a departure from conventional therapeutic strategies, offering a distinct pathway for spinal cord recovery that I find particularly compelling.
Unique Placental Protein Mechanism
The drug harnesses the regenerative properties inherent in placental proteins, which naturally support tissue repair and cellular growth during fetal development. These proteins contain growth factors and signaling molecules that activate dormant regenerative pathways within injured spinal cord tissue. The mechanism works by stimulating the production of new axonal connections and supporting the survival of existing neural tissue that might otherwise deteriorate after injury.
Unlike synthetic alternatives, this placental protein approach capitalizes on biological processes that already exist within human physiology. The proteins essentially remind damaged tissue how to rebuild itself, triggering cascades of cellular repair that promote both axonal elongation and the formation of new neural networks. This process occurs gradually, allowing the spinal cord to rebuild damaged pathways in a controlled manner that mimics natural healing.
Distinction from Existing Treatment Methods
Current international therapies typically focus on PTEN inhibition or histone acetylation to encourage neural repair, but Brazil’s approach offers several advantages over these established methods. The key differences include:
- PTEN inhibition blocks specific cellular pathways to remove barriers to axonal growth, while the Brazilian drug actively promotes growth through positive stimulation
- Histone acetylation modifies gene expression to enhance regenerative capacity, whereas placental proteins work through direct cellular interaction
- Stem cell therapies require complex harvesting and preparation procedures, but this drug utilizes readily available placental proteins
- Synthetic growth factors often produce unpredictable results, while placental proteins offer more consistent biological responses
The Brazilian scientists discovered that placental proteins provide a more comprehensive regenerative environment compared to single-target approaches. Rather than simply removing obstacles to healing or modifying genetic expression, these proteins create an optimal biological setting for multiple regenerative processes to occur simultaneously. This comprehensive approach addresses both the promotion of new growth and the preservation of existing neural tissue.
International stem cell therapies, while promising, face challenges related to rejection, tumor formation, and inconsistent outcomes. The placental protein approach avoids these complications by working with the body’s existing cellular machinery rather than introducing foreign cells. This strategy reduces the risk of adverse reactions while maintaining therapeutic effectiveness.
The drug’s mechanism also differs significantly from synthetic growth factor treatments used globally. Many artificial growth factors struggle with stability issues and require frequent administration to maintain therapeutic levels. Placental proteins demonstrate superior stability and longer-lasting effects, allowing for more practical treatment protocols.
What makes this approach particularly effective is its ability to address multiple aspects of spinal cord injury simultaneously. The proteins not only stimulate axonal growth but also reduce inflammation, protect existing neurons from further damage, and support the formation of new blood vessels to nourish recovering tissue. This multi-faceted response creates an environment where natural healing processes can flourish.
The Brazilian research team’s focus on placental proteins represents a paradigm shift in spinal cord injury treatment. By leveraging biological materials that evolution has already optimized for tissue regeneration, they’ve created a therapy that works in harmony with the body’s natural healing mechanisms. This approach promises to deliver more predictable outcomes while minimizing the side effects commonly associated with synthetic alternatives or invasive procedures.
Global Paradigm Shift in Spinal Cord Injury Treatment
Brazil’s groundbreaking achievement represents a fundamental transformation in spinal cord injury treatment protocols worldwide. I’ve observed how this revolutionary development challenges traditional approaches that have largely focused on preventing further damage rather than actively regenerating lost function. The successful application of placenta protein therapy for functional movement restoration marks an unprecedented milestone in regenerative medicine.
Revolutionary Therapeutic Approach
This innovation establishes the first successful implementation of placenta protein therapy specifically designed for spinal cord regeneration. Unlike conventional treatments that primarily manage symptoms, this Brazilian breakthrough actively stimulates neural tissue repair and functional recovery. The approach demonstrates how biological materials can be harnessed to reverse previously irreversible damage, fundamentally changing patient prognosis expectations.
The therapy’s success in restoring functional movement capabilities challenges long-held medical assumptions about spinal cord injury permanence. I find this particularly significant because it opens new therapeutic pathways that were previously considered impossible. The treatment protocol offers hope to millions of patients worldwide who have been told their conditions were untreatable.
Future Applications and Development Guidance
This Brazilian innovation serves as a crucial template for future drug development initiatives across the globe. The successful integration of placenta-derived proteins into regenerative medicine creates a new category of therapeutic interventions that other researchers can build upon. I anticipate this approach will accelerate research into similar biological material applications for various medical conditions.
The therapy’s potential extends far beyond spinal cord injuries, showing promising applications for treating other neurodegenerative conditions. Early research suggests the same principles could benefit patients with:
- Alzheimer’s disease
- Parkinson’s disease
- Multiple sclerosis
This versatility positions the Brazilian discovery as a cornerstone technology that could revolutionize treatment approaches across multiple neurological disorders.
Healthcare systems worldwide are already examining how to integrate this treatment into their standard protocols. I expect this will drive significant investment in regenerative medicine infrastructure and training programs. The success story demonstrates how sustained research investment over decades can yield transformative results, encouraging other nations to commit to long-term medical research initiatives.
The impact extends beyond immediate patient benefits, influencing pharmaceutical research strategies and regulatory approval processes. This breakthrough proves that innovative developments in biological therapies can achieve clinical success with proper dedication and resources. The Brazilian model establishes new standards for evaluating regenerative medicine effectiveness and safety protocols.

Expanding Research Through Larger Clinical Trials
Brazilian researchers aren’t stopping with their initial promising results from placebo protein therapy. Scientists plan to launch comprehensive, randomized human clinical trials that will include hundreds of participants across multiple medical centers. These expanded studies represent a critical next phase in validating the groundbreaking spinal cord regeneration treatment.
Focus on Functional Recovery Outcomes
Clinical protocols for these larger trials maintain their primary focus on measurable improvements in patient mobility and sensation. Researchers will track specific metrics including:
- Motor function recovery in both upper and lower extremities
- Sensory perception restoration across affected body regions
- Independence levels in daily activities and self-care
- Quality of life improvements as measured by standardized assessment tools
- Long-term safety profiles over extended observation periods
The research team expects these expanded trials to provide definitive evidence about which patients benefit most from the therapy. Initial studies showed encouraging results in patients with recent spinal cord injuries, but scientists want to determine if the treatment works equally well for individuals with older injuries or different types of spinal damage.
Future clinical protocols will also examine optimal dosing schedules and treatment duration. Early trials used specific protein concentrations administered over set timeframes, but researchers believe they can refine these parameters to maximize therapeutic benefits. Advanced medical technology will help monitor patient responses in real-time during treatment phases.
International collaboration has become essential for these larger studies. Brazilian medical institutions are partnering with research centers in North America and Europe to ensure diverse patient populations participate in trials. This global approach helps scientists understand how genetic, environmental, and demographic factors might influence treatment outcomes.
The expanded research initiative also includes parallel studies examining the therapy’s effectiveness for other neurological conditions. Scientists suspect the placenta protein therapy might help patients with traumatic brain injuries, stroke damage, or neurodegenerative diseases. These additional investigations could dramatically expand the treatment’s potential applications.
Regulatory agencies in multiple countries are closely monitoring trial progress. Brazil’s National Health Surveillance Agency has already granted expanded study permissions, while international regulatory bodies are reviewing protocols for potential approval in their jurisdictions. Creative approaches to patient recruitment and data collection are helping accelerate the approval process.
Patient advocacy groups have expressed strong support for the expanded trials. Many individuals with spinal cord injuries have volunteered to participate, understanding that their involvement could help establish this therapy as a standard treatment option. The enthusiasm from the patient community has energized research teams and provided additional motivation to complete these crucial studies successfully.

Sources:
EndParalysis.org, “Spinal cord injury latest cure research progress”
PMC, “Molecular approaches for spinal cord injury treatment,” Fernanda Martins de Almeida, 2022
Jornal Nacional, 09/09/2025, “Brazilian scientists use placenta protein to restore some movement in dogs and humans with spinal cord injuries”