Scientists have uncovered a remarkable discovery: the human heart contains over 40,000 specialized sensory neurons forming a “little brain” capable of processing information and communicating with the brain through electrical and biochemical signals.
Key Takeaways
- The heart’s 40,000 neurons consist of three specialized cell types — sensory, motor, and local circuit neurons — which collaboratively monitor heart rhythm, pressure, and chemical fluctuations, while autonomously controlling muscle contractions.
- Heart-brain communication is bidirectional, flowing through the vagus nerve and sympathetic pathways. The heart actively relays data to the brain, influencing memory, emotions, and decision-making capabilities.
- Six essential proteins regulate electrical communication among billions of heart cells via gap junctions. Smaller proteins guide the actions of larger ones to maintain synchronized heartbeat activity.
- Heart size influences neural complexity. Human hearts contain approximately 43,000 to 94,000 cardiac neurons, whereas mice possess only about 1,000, reflecting the more intricate neural control required for larger organs.
- A novel neuroimmune cardiovascular circuit has been identified, linking the heart’s neural network to the immune system along with organs such as the gut, liver, and adipose tissue. This discovery suggests an integrated system affecting overall health.
To Learn More
To explore further into this emerging science, you can visit this official NIH study on cardiac neurobiology, which details the heart’s neural capacity and role in health regulation.
Your Heart Contains 40,000 Neurons That Act as a “Little Brain”
Most people think of the heart as a simple pump, but I’ve discovered through scientific research that it’s far more sophisticated than anyone imagined. The human heart houses approximately 40,000 sensory neurons called sensory neurites, creating what scientists now call a “little brain” right inside your chest. This intricate neural network challenges everything we thought we knew about how the cardiovascular system operates.
The Intracardiac Nervous System Works Independently
This remarkable network, known as the intracardiac nervous system (IcNS), sits embedded in the superficial layers of the heart wall. Unlike other organs that simply follow commands from the brain, your heart’s neural network can actually think for itself. The IcNS processes local cardiac changes and controls heart function autonomously, without waiting for signals from the brain.
Think of it this way: while your brain might tell your heart to speed up during exercise, the heart’s own neural network continuously monitors and adjusts its function based on immediate local conditions. This independence allows your heart to respond instantly to changes in blood pressure, oxygen levels, or mechanical stress. The system acts as a sophisticated feedback loop, constantly fine-tuning cardiac performance in real-time.
Neural Clusters Form Sophisticated Communication Networks
Individual clusters within this cardiac neural network contain three distinct types of neurons that work together like miniature processing units. Each cluster includes:
- Sensory neurons that detect changes in heart rhythm, pressure, and chemical composition
- Motor neurons that directly control heart muscle contractions and electrical activity
- Local circuit neurons that process information and coordinate responses within each cluster
These clusters don’t work in isolation. Interganglionic neurons create communication pathways across the entire heart, allowing different regions to share information and coordinate their responses. This sophisticated communication system ensures that your heart functions as a unified, intelligent organ rather than just a mechanical pump.
Recent advances in single-cell RNA sequencing have revealed even more surprising details about cardiac neurons. Scientists have identified unexpected neuronal diversity within the heart, including specialized rhythm-regulating neurons that function similarly to pattern generators found in the central nervous system. These discoveries suggest that your heart’s neural network might be even more complex than previously understood.
The implications of this research extend far beyond basic biology. Understanding how the heart’s neural network operates could revolutionize treatments for heart disease, arrhythmias, and other cardiovascular conditions. Instead of simply addressing symptoms, future therapies might target the heart’s own neural processing capabilities.
This cardiac “little brain” also helps explain why heart transplant recipients sometimes report personality changes or new preferences. The transplanted heart brings its own neural network, complete with learned responses and processing patterns from the donor. While the science behind this phenomenon continues to evolve, it highlights just how integral the heart’s neural network is to overall function.
Modern technology increasingly recognizes the heart’s sophisticated nature. Advanced smartwatch technology now monitors heart rate variability, which reflects the constant communication between the heart’s neural network and the autonomic nervous system. These devices can detect subtle changes that might indicate stress, illness, or other health concerns.
The heart’s neural network represents a perfect example of biological engineering. Rather than relying solely on external control, evolution equipped the heart with its own intelligent processing system. This redundancy ensures that cardiac function continues even when communication with the brain is compromised, making the heart one of the most resilient organs in the human body.
How Your Heart and Brain Communicate Through a Two-Way Highway
The heart and brain don’t operate in isolation—they engage in constant, sophisticated dialogue through multiple pathways that form a bidirectional communication network. This intricate system goes far beyond the simple notion that your brain merely tells your heart what to do. Instead, both organs actively exchange information that influences everything from your emotional state to your body’s stress response.
The Vagus Nerve: Your Body’s Information Superhighway
At the center of this communication network sits the vagus nerve, the longest nerve in your body and arguably one of the most important. This cranial nerve serves as the primary conduit for information flowing between your heart and brain, carrying signals in both directions with remarkable efficiency.
Your brain influences your heart through two distinct pathways:
- Parasympathetic pathway: Utilizes vagal motor neurons that reduce your heart rate through cholinergic signaling—essentially telling your heart to slow down and relax.
- Sympathetic pathway: Employs preganglionic neurons that enhance both heart rate and contractility through adrenergic signaling, preparing your body for action or stress responses.
How Your Heart Talks Back to Your Brain
The communication doesn’t flow in just one direction. Your heart actively sends crucial information back to your brain through ascending interoceptive pathways, creating a feedback loop that keeps your body functioning optimally. This constant stream of sensory data from your heart and blood vessels initiates both peripheral and central reflexes that help maintain homeostasis.
Sympathetic afferent fibers serve as messengers, conveying signals from specialized sensors called baroreceptors and chemoreceptors. These sensors monitor blood pressure, oxygen levels, and chemical changes in your blood. The signals travel through the cardiac plexus to sympathetic chain ganglia, then enter your spinal cord via thoracic spinal nerves T1 through T5.
From your spinal cord, these signals reach the brainstem’s nucleus tractus solitarius (NTS), which functions as a primary visceral sensory processing center. The NTS integrates this cardiac information with other bodily signals, helping coordinate appropriate responses throughout your nervous system.
This sophisticated communication system explains why certain heart conditions can affect cognitive function and why stress and emotions have such immediate effects on cardiovascular health. Modern smartwatch technology has begun tapping into these signals, with some devices capable of detecting life-threatening conditions by monitoring heart rhythm patterns that reflect this heart-brain communication.
The discovery that your heart contains over 40,000 neurons reinforces the complexity of this relationship. These cardiac neurons don’t just passively relay information—they actively process and integrate signals before sending them to your brain. This local processing capability means your heart can make certain decisions independently, responding to changes in blood flow or pressure without waiting for instructions from your brain.
Understanding this bidirectional communication helps explain phenomena that might otherwise seem mysterious. The feeling of your heart “racing” during anxiety isn’t just your brain telling your heart to beat faster—it’s also your heart sending distress signals back to your brain, creating a feedback loop that can intensify emotional responses.
Scientists continue uncovering new aspects of this heart-brain connection, much like how researchers have made breakthrough discoveries about other complex neurological phenomena. Each new finding reveals additional layers of complexity in how these vital organs coordinate to keep you alive and functioning.
This intricate communication network operates continuously, adjusting and adapting to your body’s changing needs. Whether you’re sleeping, exercising, or experiencing stress, your heart and brain maintain their constant dialogue, fine-tuning your physiological responses through this remarkable two-way highway of information exchange.
Six Proteins Control Electrical Communication in Billions of Heart Cells
Scientists at the Cedars-Sinai Heart Institute have made a groundbreaking discovery that challenges everything I thought I knew about how the heart coordinates its electrical activity. Their research revealed six essential proteins that orchestrate electrical communication in heart cells through specialized structures called gap junctions, dramatically expanding upon previous knowledge that identified only one such protein.
The most fascinating aspect of this discovery lies in how these proteins work together. The smallest protein actually directs the largest one, creating a sophisticated command system that ensures synchronized activity across billions of heart cells during each heartbeat. This intricate coordination happens through a process called alternative translation, which allows multiple shorter proteins to be synthesized from the same gene — essentially creating multiple tools from a single genetic blueprint.
Critical Implications for Heart Health
This research carries profound implications for understanding heart disease. When cell communication decreases — a common occurrence in heart failure — the risk of dangerous rhythm disturbances increases significantly. These rhythm problems can prove fatal, making this discovery particularly valuable for developing new treatment approaches.
The connection between electrical signals in the brain and heart demonstrates how sophisticated cellular communication networks operate throughout the body. Both organs rely on precise electrical coordination, though the heart’s system involves billions of cells working in perfect synchrony with each beat.
Perhaps most promising is the therapeutic potential this research reveals. mTOR inhibitors, drugs already approved for immunosuppression in organ transplants, can influence this electrical coordination process. These medications boost electrical communication between heart cells and potentially prevent fatal arrhythmias by enhancing the protein networks responsible for cellular coordination.
This discovery fundamentally changes how I understand cardiac electrical activity. Rather than relying on a single protein to facilitate communication between heart cells, the heart employs a sophisticated network of six proteins working in concert. The smaller proteins act as conductors, directing larger ones to ensure every cell receives the electrical signals needed for proper heart rhythm.
The research opens new pathways for treating heart conditions, particularly those involving electrical conduction problems. By targeting the protein networks responsible for cellular communication, doctors may soon have more precise tools for preventing dangerous arrhythmias and improving outcomes for patients with heart failure.
Your Heart Influences Memory, Emotions, and Decision-Making
The heart’s remarkable network of over 40,000 sensory neurites extends far beyond basic cardiovascular function, playing a crucial role in how I process memories and make decisions. These specialized nerve cells actively participate in both short-term and long-term memory formation, creating a dynamic partnership between cardiac activity and cognitive function.
Changes in heart rhythm and electrical activity directly influence brain function through ascending neural pathways that affect my autonomic nervous system. This intricate communication system impacts endocrine balance, including the regulation of glucocorticoids and mineralocorticoids, while also affecting cardiovascular volume. Scientists have discovered that these heart-brain connections can significantly alter mood and cognitive performance.
How Heart Centers Process Information
Primary heart integration centers receive continuous input from secondary brain structures that help regulate several vital functions:
- Immunity and immune response coordination
- Stress hormone production and release
- Pain perception and response mechanisms
- Emotional processing and regulation
- Reward-driven behavior patterns
Advanced imaging techniques like PET scans and EEG monitoring have revealed the dynamic coupling between heart and brain activity. Research shows thalamic activation during myocardial ischemia, demonstrating how cardiac events immediately trigger brain responses. This connection helps explain why cardiac monitoring technology has become increasingly important for overall health assessment.
Heartbeat-evoked potentials (HEPs) provide compelling evidence of this heart-brain interaction. These electrical signals link changes in cardiac function directly with emotional stress responses, creating measurable patterns that scientists can track. Variations in HEP amplitude reflect the strength of communication between heart and brain, offering insights into how cardiac health affects mental well-being.
The implications extend beyond basic physiology. I can experience shifts in decision-making ability, memory recall, and emotional stability based on heart rhythm patterns and cardiac health. This discovery challenges traditional views that separate heart function from cognitive processes, revealing instead an integrated system where cardiac activity continuously influences brain performance and mental state.
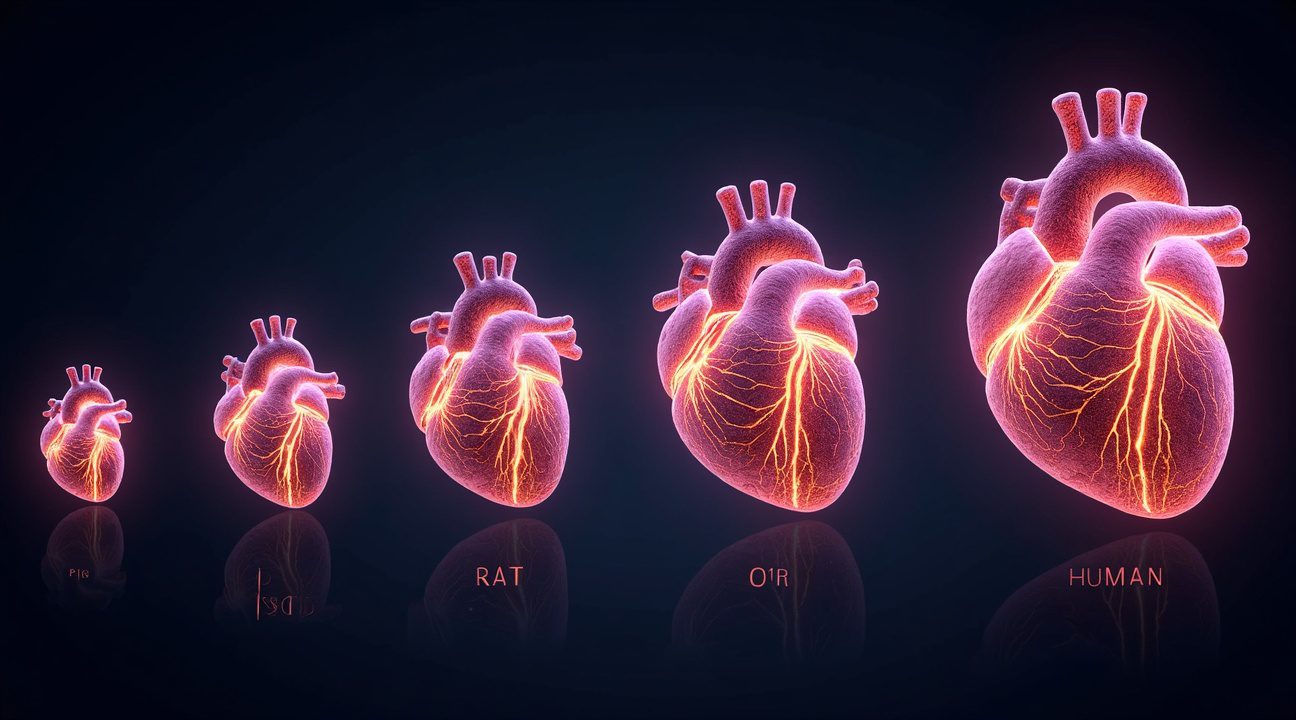
Heart Size Determines Neural Network Complexity Across Species
The intricate neural network within the heart varies dramatically across different species, with heart size serving as the primary determining factor for network complexity. This correlation reveals fascinating insights into how evolution has shaped cardiac function across the animal kingdom.
Species-Specific Neural Distribution Patterns
Research has demonstrated clear patterns in how intrinsic cardiac neurons (ICNs) are distributed among various mammals. The following species show distinct neural densities that correspond directly to their cardiac mass:
- Mice: possess approximately 1,000 ICNs within their tiny hearts
- Rats: contain roughly 6,500 specialized cardiac nerve fibers
- Pigs: house around 12,000 ICNs throughout their cardiac tissue
- Humans: maintain between 43,000 and 94,000 intrinsic cardiac neurons
These specialized nerve fibers don’t simply increase proportionally with heart size. Instead, they form sophisticated networks that regulate cardiac activity with remarkable precision. The distribution follows specific anatomical patterns, concentrating in areas where fine-tuned control becomes most critical for optimal heart function.
The heart’s ability to monitor itself represents one of nature’s most efficient self-regulation systems. Sensory signals from baroreceptors continuously track pressure changes within cardiac chambers, while chemoreceptors detect shifts in blood chemistry and temperature. This constant surveillance allows the heart to respond instantly to physiological demands without waiting for commands from the central nervous system.
I find it particularly striking how larger hearts require exponentially more neural infrastructure to maintain proper function. A human heart doesn’t just need more neurons than a mouse heart—it needs a completely different organizational structure. The complexity increases not just in quantity but in the sophistication of neural pathways and communication networks.
The diversity among ICN populations extends beyond simple numbers. Different species have evolved unique organizational patterns that reflect their specific physiological needs. For instance, deep-sea creatures likely possess specialized cardiac neural adaptations that help them survive extreme pressure conditions.
These findings underscore the essential role that intrinsic cardiac neurons play in rhythm regulation. The heart’s neural network operates as an independent control system, capable of maintaining basic cardiac function even when connections to the brain are severed. This autonomy explains why heart transplants can function successfully despite being disconnected from the recipient’s nervous system during surgery.
The implications for understanding heart disease become clearer when considering how disruptions to these neural networks might contribute to cardiac dysfunction. If the heart relies so heavily on its internal neural communication system, damage to these pathways could explain various arrhythmias and other cardiac conditions that don’t have obvious structural causes.
Modern cardiac research increasingly focuses on how these neural networks might be targeted for therapeutic interventions. Understanding the precise mechanisms through which ICNs communicate could lead to new treatments that work with the heart’s natural regulatory systems rather than against them.
The electrical and biochemical signals that flow through these cardiac neural networks create a constant dialogue between heart and brain. This communication occurs through multiple pathways, including the vagus nerve and sympathetic nervous system connections. However, the heart’s intrinsic neural network can override external commands when necessary, prioritizing immediate cardiac needs over broader physiological demands.
Scientists continue investigating how environmental factors and lifestyle choices might influence the development and function of these cardiac neural networks. The research suggests that maintaining heart health involves more than just keeping arteries clear—it requires preserving the integrity of these complex neural communication systems that keep our hearts beating with remarkable consistency throughout our lives.
The Neuroimmune Cardiovascular Circuit Connects Heart to Immune System and Other Organs
Recent discoveries have revealed something remarkable about how our bodies function: the heart doesn’t operate in isolation. I’ve learned that scientists now support a groundbreaking hypothesis called the neuroimmune cardiovascular circuit, which fundamentally changes how we understand heart health and disease.
This new framework connects the cardiovascular brain circuit with immune function and several peripheral systems, including the gut, liver, and adipose tissue. Brain effector neurons within the cardiovascular network don’t just send signals to the heart – they also communicate with these peripheral organs, creating an intricate web of interconnected regulation that scientists are just beginning to understand.
A Shift from Traditional Thinking
For years, medical professionals viewed the brain as the primary controller of heart function. This new evidence presents a paradigm shift from that traditional perspective. Instead of seeing the brain as the sole commander, researchers now recognize an anatomically linked network that operates through the autonomic nervous system.
The heart’s 40,000 neurons play a crucial role in this expanded understanding. These specialized cells don’t just process local information – they participate in a complex communication system that involves multiple organ systems working together in ways scientists hadn’t previously recognized.
Co-regulation and Mutual Dependence
This neuroimmune cardiovascular circuit emphasizes something critical: co-regulation and mutual dependence between the body’s key systems. The heart influences immune function, while the immune system affects cardiovascular health. Similarly, signals from the gut, liver, and fat tissue all contribute to this regulatory network.
Consider how this applies to everyday health scenarios. When someone experiences stress, the response isn’t limited to increased heart rate. The entire circuit activates, affecting immune function, digestive processes, and metabolic activity. This helps explain why cardiovascular disease often appears alongside other health conditions – they’re all part of the same interconnected system.
Modern technology has enabled researchers to map these connections with unprecedented precision. Just as advanced monitoring devices can track multiple health parameters simultaneously, scientists can now observe how different organ systems communicate in real-time.
This expanded understanding opens new possibilities for treating cardiovascular disease. Rather than focusing solely on the heart, healthcare providers might address the entire circuit, potentially improving outcomes by supporting the immune system, optimizing gut health, or addressing metabolic dysfunction. The neuroimmune cardiovascular circuit represents a comprehensive approach that acknowledges the sophisticated nature of human physiology and the interconnectedness that defines our biological systems.
Sources:
American Heart Association – “Intrinsic Cardiac Nervous System: Neuromodulation of Cardiovascular Function and Implication for Heart Failure Therapy”
Cedars-Sinai – “Researchers Identify Key Proteins Responsible for Electrical Communication in the Heart”
National Center for Biotechnology Information (NCBI) – “Intrinsic Cardiac Nervous System: Role in Cardiac Control and Neurological Disease”
Harvard Medical School – “Heart-Brain Health Is a Two-Way Street”
AIMS Neuroscience – “The Intrinsic Cardiac Nervous System and its Role in Cardiologic and Neurologic Diseases”
Nature Communications – “Single-cell transcriptomic and spatial atlas of the adult murine intrinsic cardiac nervous system”
Karolinska Institutet – “The Heart Has Its Own ‘Brain'”
Thomas Jefferson University – “The Heart’s ‘Little Brain'”