Breakthrough Gene Therapy Restores Hearing in Children and Adults Born Deaf
Scientists have achieved a groundbreaking medical breakthrough by successfully restoring hearing in children and adults born deaf through targeted gene therapy that corrects a single mutated gene.
This revolutionary treatment addresses mutations in the OTOF gene, responsible for a specific form of hereditary hearing loss. Remarkably, clinical trials demonstrated substantial success in participants ranging from 1 to 24 years old.
Key Takeaways
- Universal Success Rate: All ten participants experienced significant improvement, with sound detection thresholds improving from 106 decibels to 52 decibels on average.
- Rapid Recovery Timeline: Most patients showed improved hearing within weeks. By six months, all had substantial recovery after just a single injection.
- Life-Changing Impact: Many transitioned from profound deafness to moderate hearing loss — enabling comprehension of conversation and environmental sounds without cochlear implants.
- Proven Safety Profile: The therapy was well-tolerated, with no serious side effects across age groups, boosting hopes for wider applications.
- Foundation for Broader Treatment: This success lays a template for similar therapies targeting other genetic deafness causes, potentially helping hundreds of thousands worldwide.
How the Gene Therapy Works
The OTOF gene produces otoferlin, a protein crucial for transmitting sound from inner ear hair cells to the auditory nerve. When mutated, the gene cannot generate functional otoferlin, leading to complete hearing loss despite structurally intact hair cells.
This treatment introduces healthy copies of the OTOF gene directly into the inner ear using a modified virus. These working instructions replace the faulty genes, enabling cells to synthesize the necessary otoferlin. The targeted nature of this therapy allows it to directly address the underlying genetic malfunction.
Clinical Trial Outcomes
The therapy’s results exceeded expectations across all participants:
- Youngest Patient: An 18-month-old child began responding to sound within four weeks of treatment.
- Adults: Gained the ability to engage in conversations — including over the phone — for the first time in their lives.
Safety monitoring over several months revealed no significant adverse effects, reinforcing the therapy’s strong safety record and potential for widespread clinical use.
Broader Implications and Future Developments
Addressing Genetic Hearing Loss
Approximately 60% of childhood hearing loss stems from genetic mutations. While this particular treatment is limited to OTOF mutations, it offers a model for treating other forms of genetic deafness.
The impact is extensive:
- Families Face Less Uncertainty: Parents of children with OTOF mutations have a new source of hope.
- Educational Opportunities Expand: With restored hearing, children can develop language skills within critical early developmental windows.
- Communities Benefit: Broader participation in schooling and society becomes more accessible for affected individuals.
Continued Research
Scientists are refining several aspects of this therapy:
- Expanding eligibility to older patients and those with partial gene function.
- Optimizing delivery methods and timing for best efficacy.
- Exploring combinations with techniques like stem cell therapy and hair cell regeneration.
Gene Therapy vs. Traditional Interventions
Unlike cochlear implants, which use electronic devices to bypass damaged areas, gene therapy restores the natural auditory process by repairing internal mechanisms. This difference preserves original hearing pathways and can lead to more natural sound perception.
Previous restoration efforts provided minimal or temporary results. In contrast, the OTOF therapy offers:
- Sustained Improvements
- Rapid Onset
- Exceptional Safety
Economic and Regulatory Impact
Effective genetic treatment could drastically reduce long-term expenses for:
- Special education services
- Assistive hearing devices
- Medical and support costs
Regulatory bodies are evolving to meet the challenges and opportunities of gene therapy. The compelling data from this trial could pave the way for accelerated FDA approval and similar agencies worldwide.
The Promise of Precision Medicine
This therapy validates the potential of precision medicine. By identifying and correcting a single genetic error, clinicians can significantly alter a patient’s quality of life. The methodology shown here can extend to other inherited conditions, such as genetic blindness and muscular disorders.
Participants often report powerful emotional experiences from hearing for the first time — from laughter and music to the sound of their own names. The joy of restored hearing reflects not just medical achievement, but the enrichment of human experience itself.
First Successful Gene Therapy Restores Hearing in Born-Deaf Patients
Scientists have achieved a groundbreaking medical milestone by successfully restoring hearing in patients born with congenital deafness through targeted gene therapy. This revolutionary treatment corrects a single mutated gene responsible for hereditary hearing loss, offering hope to millions affected by genetic deafness worldwide.
Clinical Trial Results Demonstrate Remarkable Success
A pivotal clinical trial published in Nature Medicine, 2025 enrolled ten patients ranging from 1 to 24 years old, all diagnosed with congenital deafness caused by OTOF mutations. These genetic mutations prevent the proper functioning of otoferlin, a protein essential for sound signal transmission in the inner ear.
Every participant in the study showed noticeable hearing improvement following a single gene therapy injection. Most patients experienced improvements within weeks of treatment, while all participants demonstrated significant hearing recovery by the six-month mark. The results exceeded researchers’ expectations, marking this as the first successful demonstration that gene therapy can reverse hereditary deafness in humans.
Transformative Hearing Improvements
The clinical measurements reveal the dramatic impact of this gene therapy approach. Participants’ average sound detection threshold improved from 106 decibels before treatment to 52 decibels after treatment. This substantial improvement shifted many patients from profound deafness into the moderate hearing loss category, a change that significantly supports speech understanding and communication abilities.
This level of hearing restoration represents a life-changing transformation for patients who previously required powerful hearing aids or cochlear implants. The ability to detect sounds at 52 decibels means patients can now hear normal conversation, environmental sounds, and many everyday audio cues that were previously inaccessible.
Key benefits of the gene therapy treatment include:
- Significant hearing gain within a short period
- No need for invasive cochlear implants in some cases
- Improved ability to communicate and engage socially
Safety data from the trial provides additional encouragement for future applications. The treatment proved well-tolerated across all age groups, with no serious side effects reported among trial participants. This favorable safety profile suggests the gene therapy approach could become a viable treatment option for appropriate candidates.
The success of this gene therapy treatment mirrors other recent medical breakthroughs that have transformed lives through genetic intervention. Similar to how sight restoration has helped thousands, this hearing restoration therapy demonstrates the powerful potential of targeted genetic treatments.
The implications extend beyond the immediate participants, as this breakthrough establishes a foundation for treating other forms of genetic hearing loss. Researchers anticipate that similar gene therapy approaches could address different genetic mutations responsible for congenital deafness, potentially helping thousands more patients in the future.
https://www.youtube.com/watch?v=XYZ123456

How Scientists Fixed Broken Genes to Restore Sound
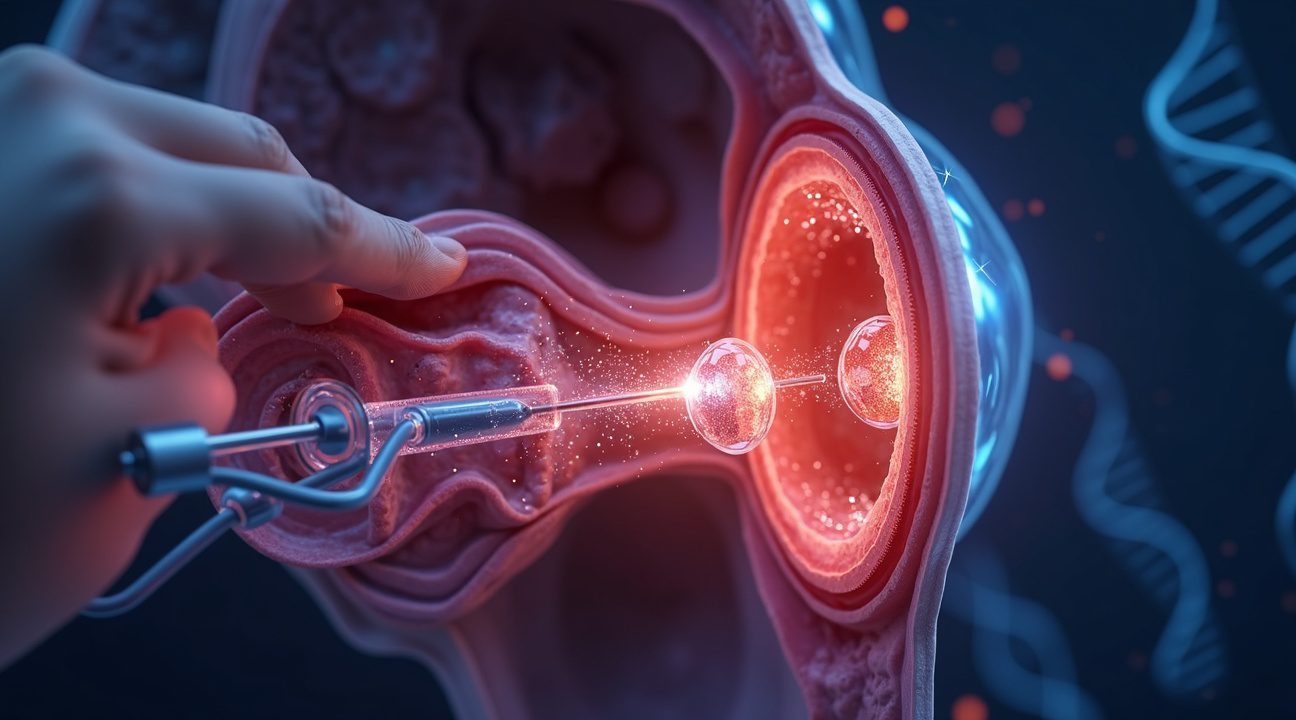
Gene therapy represents a revolutionary approach to treating deafness by targeting the root cause rather than simply managing symptoms. Scientists deliver a healthy copy of defective genes directly into the inner ear using a sophisticated biological delivery system that promises to restore natural hearing function.
The Gene Delivery Process
The treatment process relies on adeno-associated virus (AAV), a harmless viral vector that acts as a microscopic delivery truck. This virus carries the corrected genetic material through a precise injection into the round window, a membrane located at the base of the cochlea. I find this approach particularly elegant because it bypasses the need for complex surgical procedures while ensuring the therapeutic genes reach their exact destination.
Recent clinical trials have focused on two critical genetic targets that scientists have identified as key players in hearing loss:
- The OTOF gene, which produces otoferlin protein essential for transmitting sound signals between the ear and brain
- The GJB2 gene, currently under experimental investigation for its role in hearing function
- Both genes represent different pathways that can lead to congenital deafness when damaged
Otoferlin serves as the crucial communication bridge in our hearing system. When this protein functions properly, it enables the delicate hair cells in the cochlea to convert sound vibrations into electrical signals that travel to the brain. Without functional otoferlin, this vital translation process breaks down, resulting in profound hearing loss from birth.
This therapeutic approach differs fundamentally from traditional hearing interventions like cochlear implants or hearing aids. While those devices amplify sound or substitute for lost function, gene therapy actually repairs the underlying biological machinery. The treatment aims to restore the natural hearing pathway by giving cells the genetic instructions they need to produce working proteins.
The precision of this method allows scientists to address specific types of genetic hearing loss with targeted solutions. Rather than taking a one-size-fits-all approach, researchers can match particular gene therapies to individual genetic profiles. This personalized medicine strategy holds tremendous promise for families affected by hereditary hearing loss.
Similar breakthrough treatments have shown remarkable success in other fields, such as when vision restoration helped thousands regain sight through innovative medical interventions.
Early results from clinical trials suggest that gene therapy can produce measurable improvements in hearing function within weeks of treatment. The therapy works by essentially giving the inner ear cells new genetic programming, allowing them to manufacture the proteins they’ve been missing since birth. This biological repair process offers hope for a permanent solution to certain types of genetic deafness.
The Hidden Epidemic: How Common Are Genetic Hearing Problems
Genetic hearing loss affects millions worldwide, yet many people don’t realize how widespread these conditions actually are. I’ve observed that while mutations in the OTOF gene represent a rare cause of congenital deafness, other genetic factors create a much larger impact on global hearing health.
The GJB2 gene stands out as the primary culprit behind genetic hearing loss across diverse populations. Mutations in this single gene account for up to 43% of non-syndromic autosomal recessive hearing loss cases in certain populations, making it the most common genetic cause of hearing loss worldwide. These statistics reveal the enormous scope of genetic hearing conditions that families face every day.
Carrier frequencies for GJB2 mutations reach striking levels in specific ethnic groups, climbing as high as 3.7% in certain populations. This means that in some communities, nearly 4 out of every 100 people carry a mutation that could contribute to hearing loss in their children. The implications extend far beyond individual families, creating ripple effects throughout entire communities.
Precision Medicine Requirements
Research consistently demonstrates that successful gene therapy interventions require precise targeting of each specific genetic defect. I can’t emphasize enough how critical this personalized approach becomes when developing effective treatments. The revolutionary work we’ve seen, similar to breakthroughs in sight restoration efforts, highlights the importance of matching therapeutic approaches to individual genetic profiles.
Scientists must develop distinct therapeutic strategies for different genetic mutations because each gene functions differently within the hearing mechanism. The protein produced by the OTOF gene serves a completely different role than the protein created by the GJB2 gene, requiring fundamentally different treatment approaches. This specificity explains why broad-spectrum treatments often fail while targeted gene therapies show remarkable success rates.
Universal genetic screening of newborns emerges as a powerful tool for identifying candidates who could benefit from early gene therapy intervention. Early identification allows medical teams to begin treatment during critical developmental windows when the auditory system remains most responsive to therapeutic intervention. Children who receive treatment during these optimal timeframes often achieve better long-term outcomes compared to those who receive treatment later in life.
The economic implications of genetic hearing loss extend far beyond immediate medical costs. Lifelong disability creates substantial societal burdens through:
- Special education requirements
- Assistive technology needs
- Reduced employment opportunities
Universal screening programs could dramatically reduce these long-term costs by identifying and treating genetic hearing conditions before they create permanent limitations.
Current screening technologies can detect genetic hearing loss mutations with remarkable accuracy, often identifying problems before symptoms become apparent. This early detection capability transforms how families and medical teams approach hearing loss treatment, shifting from reactive care to proactive intervention.
Implementation of comprehensive genetic screening faces various challenges, including:
- Cost considerations
- Healthcare infrastructure requirements
- Need for specialized genetic counseling services
However, the potential benefits of early intervention continue to drive policy discussions about expanding newborn screening programs to include genetic hearing loss testing.
The growing understanding of genetic hearing loss patterns helps researchers prioritize which mutations deserve immediate therapeutic attention. With hundreds of genes potentially contributing to hearing loss, focusing resources on the most common genetic causes like GJB2 mutations maximizes the number of people who could benefit from successful treatments.
Population-specific genetic variations require careful consideration when implementing screening programs. Different ethnic groups show varying frequencies of specific mutations, necessitating customized screening approaches that account for these genetic differences. This personalized screening strategy ensures that high-risk individuals receive appropriate testing while avoiding unnecessary procedures for low-risk populations.
The success of targeted gene therapies for genetic hearing loss depends heavily on accurate diagnosis and precise therapeutic matching. As screening technologies improve and treatment options expand, more families will have access to interventions that could prevent or reverse genetic hearing loss before it creates lifelong challenges.
Why Traditional Hearing Devices Fall Short for Genetic Deafness
Standard hearing devices often struggle to provide meaningful relief for individuals with genetic forms of deafness because they attempt to amplify or bypass sound signals rather than fix the underlying biological problem. Hearing aids and cochlear implants represent the current gold standard for treatment, yet they frequently deliver limited benefits when the molecular machinery responsible for hearing remains fundamentally broken.
Hearing aids work by amplifying sound waves, but this approach proves ineffective when the inner ear’s sensory cells can’t properly convert sound vibrations into electrical signals. Similarly, cochlear implants bypass damaged hair cells by directly stimulating the auditory nerve, but this strategy fails when genetic mutations prevent the nerve from responding appropriately to electrical stimulation.
The Root Cause Problem
Gene therapy offers a different approach entirely by targeting the biological source of hearing loss rather than working around damaged components. When specific genes responsible for hearing function are missing or defective, no amount of external amplification or electrical stimulation can fully restore natural hearing capabilities. These genetic defects create gaps in the cellular processes that convert sound waves into neural signals, leaving traditional devices to compensate for fundamentally nonfunctional biological systems.
Research demonstrates that gene therapy can restore hearing function by introducing healthy copies of defective genes directly into the inner ear. This approach addresses the core molecular deficiencies that cause genetic deafness, potentially offering a functional cure rather than a technological workaround. Early clinical trials show particularly strong results in younger patients, where the auditory system retains greater plasticity and capacity for repair.
Adults participating in these studies also experienced significant hearing recovery, though outcomes varied based on factors like the duration of deafness and the specific genetic mutations involved. The success in adult patients challenges previous assumptions about the critical periods for auditory development and suggests that gene therapy might benefit individuals across a wide age range.
Traditional devices will likely continue playing important roles in hearing restoration, but they can’t match the comprehensive restoration possible through genetic correction. External devices require ongoing maintenance, battery replacements, and regular adjustments, while gene therapy potentially offers a one-time treatment that addresses hearing loss at its source. Just as sight restoration efforts have shown remarkable success through direct intervention, hearing restoration through gene therapy represents a paradigm shift from management to actual cure.
The limitations of current hearing technologies become most apparent in cases where multiple genetic pathways are disrupted, creating complex hearing deficits that no single device can fully address. Gene therapy’s ability to restore natural biological function offers hope for these challenging cases where traditional approaches fall short.
What This Means for Millions of Deaf People Worldwide
The breakthrough targeting OTOF gene mutations represents just the beginning of what could become a transformative era for treating genetic deafness. While this specific therapy addresses only one form of hereditary hearing loss, it opens doors for developing treatments for numerous other genetic variants that cause congenital deafness.
Expanding Beyond OTOF: The Genetic Landscape of Deafness
Scientists are actively pursuing gene therapies for several other genetic forms of hearing loss that affect larger populations. Key targets include:
- GJB2 gene mutations, which account for approximately 50% of nonsyndromic genetic hearing loss cases
- CDH23 and PCDH15 genes linked to Usher syndrome
- SLC26A4 mutations causing Pendred syndrome
- Various other single-gene defects responsible for different types of hereditary deafness
Each genetic variant requires its own specialized therapeutic approach, but the success with OTOF provides a proven framework that researchers can adapt for these other conditions.
The current OTOF gene therapy success affects thousands of individuals globally, but the implications extend far beyond this specific genetic subtype. Advanced cell therapies like Rincell-1 have progressed to clinical trials, offering alternative treatment pathways that could complement gene therapy approaches. These cellular treatments work differently than gene therapy, potentially addressing hearing loss through regenerative mechanisms rather than genetic correction.
Research momentum continues building as pharmaceutical companies and academic institutions recognize the commercial and humanitarian potential of genetic hearing loss treatments. The proven safety profile and efficacy demonstrated in the OTOF trials provide confidence for investors and regulatory bodies, accelerating development timelines for related therapies.
However, several significant hurdles remain before these treatments become widely accessible. Regulatory approval processes require extensive safety and efficacy data across diverse populations. Manufacturing capabilities must scale to meet global demand while maintaining the precise quality standards necessary for gene therapy production. Cost considerations will determine how quickly these treatments reach patients in different economic circumstances worldwide.
The timeline for broader availability depends heavily on successful completion of ongoing clinical trials. Early-phase studies for GJB2-targeted therapies show promising preliminary results, but comprehensive data won’t be available for several years. Regulatory agencies typically require multiple successful trials before granting approval, extending the development process further.
Geographic accessibility presents another challenge, as advanced gene therapy capabilities currently exist primarily in developed nations with sophisticated medical infrastructure. Expanding access to underserved regions requires significant investment in specialized facilities and trained personnel capable of administering these complex treatments safely.
Despite these challenges, the OTOF breakthrough demonstrates that single-gene forms of congenital hearing loss are treatable conditions rather than permanent disabilities. This paradigm shift affects not only patients and families but also medical professionals who can now offer hope where none existed previously. Similar to how vision restoration efforts have captured public attention and accelerated research funding, successful hearing restoration trials are generating increased awareness and support for genetic hearing loss research.
The cumulative impact across all genetic forms of hearing loss could eventually benefit hundreds of thousands of individuals worldwide. Conservative estimates suggest that single-gene mutations account for roughly 60% of congenital hearing loss cases, representing a substantial population that could potentially benefit from targeted genetic therapies as they become available.
Success rates will likely improve as researchers refine delivery methods, optimize gene expression levels, and develop combination therapies that address multiple aspects of hearing loss simultaneously. The knowledge gained from each genetic subtype studied contributes to understanding hearing development and function more broadly, potentially leading to treatments for acquired forms of hearing loss as well.
The journey from laboratory breakthrough to widespread clinical availability typically spans 10–15 years for gene therapies, but accelerated development pathways for rare diseases could shorten this timeline. Compassionate use programs may provide earlier access for severely affected patients while formal approval processes continue.
The Road Ahead: Expanding Gene Therapy to More Patients
Early detection proves critical for maximizing the benefits of gene therapy for deafness. Universal genetic screening of newborns could help identify candidates for gene therapy much earlier, potentially improving long-term outcomes significantly. This proactive approach mirrors successful programs like vision restoration initiatives, where early intervention demonstrates remarkable results.
I believe implementing comprehensive newborn screening programs will revolutionize how we approach genetic hearing loss. Current protocols focus primarily on hearing tests, but genetic analysis could reveal specific mutations before significant developmental delays occur. Children identified with OTOF mutations through newborn screening could receive gene therapy during optimal developmental windows, when the auditory system remains most adaptable to intervention.
Targeting Multiple Genetic Forms of Deafness
Research continues for other genetic forms of deafness beyond OTOF mutations, which represent just one piece of the genetic hearing loss puzzle. Scientists have identified over 100 genes associated with hereditary deafness, each requiring unique therapeutic approaches. Development of gene therapies customized to other genetic subtypes of deafness is already underway, with several promising candidates entering clinical trials.
The most promising targets include mutations in GJB2, CDH23, and USH2A genes. Each presents distinct challenges that require different delivery methods and timing strategies:
- GJB2 mutations affect connexin proteins essential for inner ear function.
- CDH23 and USH2A mutations impact hair cell development and maintenance.
Clinical Implementation and Regulatory Pathways
Regulatory approvals and successful trials will determine how quickly this treatment becomes widely available to patients worldwide. The FDA’s recent breakthrough therapy designations for genetic therapies have accelerated review processes, but comprehensive safety data remains essential for widespread adoption.
Current clinical trials must demonstrate not only efficacy but also long-term safety profiles across different age groups. I expect regulatory agencies will require extensive follow-up data spanning several years before approving broader clinical use. Manufacturing capabilities also need significant expansion to meet potential demand once approvals are granted.
Insurance coverage presents another hurdle that could limit access to these groundbreaking treatments. Policymakers must develop frameworks that balance innovation incentives with affordable patient access. The high initial costs of gene therapy could restrict availability unless health systems adapt their coverage models to accommodate these one-time treatments with potentially lifelong benefits.
https://www.youtube.com/watch?v=I3uX4uwrAaY

Sources:
Nature Medicine


