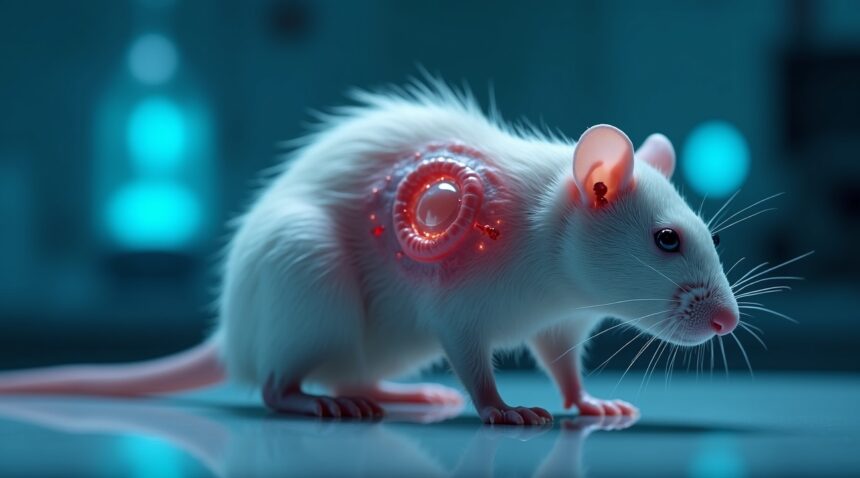
Scientists in China have achieved a remarkable breakthrough in regenerative medicine by successfully engineering a functional human ear on a rat’s back using stem cell technology and biodegradable scaffolding materials.
Key Takeaways
- Researchers at the University of Tokyo and Kyoto University successfully grew a living human ear using induced pluripotent stem cells (iPSCs) through a two-month implantation process on a rat’s back.
- The breakthrough employs entirely biological materials using dissolvable scaffolding, eliminating the risk of complications caused by permanent synthetic structures used in previous methods.
- Key regenerative components identified: scientists found retinoic acid and the Aldh1a2 gene to be crucial for tissue regeneration, hinting at the reactivation of natural healing traits lost during mammalian evolution.
- Clinical trials for facial reconstruction may commence within five years, providing new hope for individuals with congenital ear deformities or those suffering from traumatic injuries.
- Broader medical implications exist for this advancement, with the potential to construct other human organs and tissues via similar bioprinting and stem cell-based strategies.
What This Means for Regenerative Medicine
This development marks a significant milestone in the field of tissue engineering and regenerative therapies. By using human-derived cells and biodegradable materials alone, researchers are paving the way for safer, more natural solutions for reconstructive surgery.
To learn more about stem cell technologies and their broader applications in medicine, you can explore this Nature article on stem cell research.
Scientists Successfully Grow Living Human Ear Using Stem Cells and Rats
Researchers at the University of Tokyo and Kyoto University have achieved a remarkable breakthrough in regenerative medicine by successfully growing a living adult human ear on a rat’s back. This groundbreaking experiment demonstrates the incredible potential of induced pluripotent stem cells (iPSCs) in creating functional human tissue for transplantation purposes.
The scientific process begins with carefully cultured stem cells that researchers transform into ear cartilage through precise laboratory techniques. Scientists then place this specialized cartilage into a biological mold crafted to match the exact shape and dimensions of a human ear. The intricate procedure requires implanting this ear-shaped mold beneath the skin of a live rat, where it remains for approximately two months.
The Revolutionary Growth Process
During the two-month implantation period, fascinating biological processes unfold beneath the rat’s skin. The biological mold gradually dissolves while the cartilage continues to mature and develop into a fully functional ear structure. Blood vessels from the rat’s circulatory system integrate with the growing tissue, providing essential nutrients and oxygen that support healthy development. This symbiotic relationship between the host animal and the growing human tissue represents a significant advancement in tissue engineering methods.
The resulting ear doesn’t just look like human tissue – it functions as living, breathing human cartilage that could potentially be transplanted into patients requiring ear reconstruction. Scientists report that these laboratory-grown ears maintain all the structural integrity and flexibility of natural human ears, making them viable candidates for surgical implantation.
One of the most promising aspects of this research lies in its application for pediatric patients. Children who receive these stem cell-grown ears can expect the transplanted tissue to grow alongside their natural development, eliminating the need for multiple surgeries as they mature. This characteristic addresses a major limitation in current ear reconstruction procedures, where static implants often require replacement or adjustment as children grow.
Current ear reconstruction methods typically involve invasive surgical procedures that require surgeons to remove cartilage from a patient’s ribs. This approach not only creates additional surgical sites and potential complications but also limits the amount of available material for reconstruction. The new stem cell technique could completely eliminate these drawbacks by providing an unlimited supply of patient-specific ear tissue grown in laboratory conditions.
The implications of this research extend far beyond ear reconstruction alone. The successful cultivation of complex human tissue using artificial intelligence techniques and stem cell technology opens doors for growing other body parts and organs. Scientists believe similar methods could eventually produce skin grafts, nose cartilage, and even more complex organs for transplantation.
The use of iPSCs in this process offers particular advantages over other stem cell types:
- They can be derived from the patient’s own tissue, reducing the risk of immune rejection.
- They are highly versatile and can be programmed to develop into nearly any type of human tissue.
This advancement represents a significant step forward in addressing congenital ear deformities, traumatic injuries, and cancer-related ear removal cases. Patients who previously faced limited reconstruction options now have access to a potentially superior alternative that promises better aesthetic and functional outcomes.
The research team’s success builds upon decades of tissue engineering research and demonstrates how collaborative efforts between major universities can accelerate medical breakthroughs. The combination of advanced stem cell techniques with innovative implantation methods has created a reproducible process that other research institutions can adapt and refine.

How This Breakthrough Compares to Previous Ear Growing Attempts
This achievement represents the latest milestone in a fascinating journey of tissue engineering that stretches back over a decade. I’ve been following these developments, and each breakthrough builds upon previous successes while addressing critical limitations.
Early Attempts and Their Limitations
Back in 2013, researchers at Massachusetts General Hospital made headlines with their groundbreaking work using bovine collagen and sheep cells. They constructed their ear using a titanium wire frame for structural shape, which provided excellent support but created potential long-term complications. The permanent metal framework raised concerns about biocompatibility and the body’s ability to fully integrate the artificial structure.
This approach, while innovative, highlighted a fundamental challenge in tissue engineering. Permanent synthetic materials often trigger immune responses or create barriers to natural tissue integration. Patients might face ongoing complications from foreign materials remaining in their bodies indefinitely.
Revolutionary Approaches and Modern Innovations
The Japanese study takes a dramatically different approach by utilizing only human-derived cells and a dissolvable biological mold. This method eliminates the need for permanent structural frames, allowing the body to naturally absorb the scaffolding material as the tissue develops. The biological compatibility represents a significant advancement over earlier titanium-based designs.
Other researchers have explored equally creative solutions to ear reconstruction challenges:
- London-based teams successfully grew a nose on a patient’s arm, demonstrating the potential for using different body locations as growth sites.
- 3D printing technology has opened new possibilities for creating artificial intelligence-guided tissue structures.
- Scientists have also experimented with 3D-printing human ears from cow ear cells, implanting these onto printed molds and testing them in rats for up to three months. These studies provided valuable data about tissue viability and integration processes, though they still relied on synthetic molds for structural support.
The current Chinese breakthrough stands out because it may offer a more biocompatible and natural approach to tissue engineering. By using human cells exclusively and eliminating permanent foreign materials, this method addresses many concerns that plagued earlier attempts. The dissolvable scaffolding allows for complete biological integration while maintaining the structural integrity needed during the critical growth phase.
Each of these approaches has contributed essential knowledge to the field, from understanding cellular behavior to perfecting growth environments. The evolution from permanent synthetic frameworks to fully biological systems represents a clear progression in the field’s sophistication and understanding of human tissue development.
Scientists Unlock the Genetic Secret Behind Mammalian Ear Regeneration
Researchers have finally cracked the code behind mammalian tissue regeneration, discovering that the key lies in a single crucial substance: retinoic acid. This breakthrough emerged from studies published in June 2025 in the journal Science, revealing why mammals lose their ability to regenerate damaged tissue and how scientists can restore it.
The Retinoic Acid Connection
The limited ability of mammals to produce retinoic acid has long puzzled scientists studying tissue regeneration. Unlike amphibians that can regrow entire limbs, mammals typically form scar tissue instead of regenerating damaged organs. Recent research shows that activating the Aldh1a2 gene, which synthesizes retinoic acid, can completely change this outcome.
When researchers activated specific genetic switches or provided supplemental retinoic acid to test subjects, they observed remarkable results. Mice, rats, and rabbits successfully regenerated damaged ear pinnae, including complex cartilage structures that normally don’t heal properly in mammals. This process essentially turns back the evolutionary clock, restoring capabilities that mammals possessed millions of years ago.
Breakthrough Applications and Future Implications
The discovery opens unprecedented possibilities for medical treatments and regenerative medicine. Scientists can now trigger mammalian regeneration pathways that were previously considered permanently lost through evolution. The research demonstrates several practical approaches to achieving tissue regeneration:
- Direct genetic activation of the Aldh1a2 gene through targeted therapy
- Supplemental retinoic acid administration to stimulate natural regeneration processes
- Combination treatments that enhance vitamin A metabolism for sustained regeneration
- Targeted applications for specific tissue types, particularly cartilage repair
This advancement could revolutionize treatments for various conditions, from battlefield injuries to congenital defects. The ability to regenerate ear tissue represents just the beginning of what might be possible with this genetic switch approach. While artificial intelligence paving the way for the future has captured public attention, this biological breakthrough offers tangible solutions for immediate medical challenges.
The research team’s success with cartilage regrowth particularly excites medical professionals, as cartilage damage is notoriously difficult to treat using conventional methods. Unlike other tissues that can partially heal, cartilage rarely regenerates naturally in adult mammals, making this discovery especially significant for orthopedic applications.
Scientists believe this genetic approach could extend beyond ear regeneration to other organs and tissues. The Aldh1a2 gene appears to be a master regulator of regenerative processes, suggesting that similar techniques might work for liver tissue, skin repair, and even nerve regeneration. Early experiments indicate that the retinoic acid pathway influences multiple aspects of tissue development and repair.
The evolutionary biology implications are equally fascinating. Mammals apparently didn’t lose their regenerative abilities entirely but rather switched them off during evolution. This suggests that the genetic machinery for regeneration remains intact in mammalian DNA, waiting for the right trigger to reactivate it. Understanding why evolution favored scar formation over regeneration in mammals could lead to even more sophisticated therapeutic approaches.
Current research focuses on refining the delivery methods for retinoic acid and determining optimal dosages for different tissue types. Scientists are also investigating whether the regenerative effects can be sustained long-term without continuous treatment. The goal is developing practical medical applications that harness these discoveries for human patients while minimizing potential side effects.
https://www.youtube.com/watch?v=9MZh4wiVRt7abM
Clinical Trials Could Begin Within Five Years for Facial Reconstruction
The groundbreaking ear-growing technology developed by Chinese researchers represents a major advancement in reconstructive medicine, particularly for patients facing facial deformities and traumatic injuries. I believe this innovation could fundamentally change how medical professionals approach treatment for congenital conditions like microtia, where the external ear fails to develop properly during fetal growth.
Scientists working on this project anticipate that clinical trials testing this regenerative approach could commence within the next five years. This timeline reflects both the promising results from animal studies and the careful progression needed to ensure safety for human applications. The potential extends far beyond ear reconstruction, as researchers suggest these same tissue engineering techniques might adapt to regenerate other vital body parts, including windpipes and potentially more complex organs.
Overcoming Technical and Medical Hurdles
Several significant challenges remain before this technology reaches patients in clinical settings. Achieving precise ear shapes that match individual patient anatomy requires sophisticated bioengineering techniques that can replicate the intricate cartilage structures of human ears. Scientists must also solve the complex problem of integrating nerves and blood vessels into the grown tissue, ensuring that reconstructed ears can function properly and maintain healthy blood flow.
Immune compatibility presents another critical hurdle, as researchers work to prevent the body from rejecting the newly grown tissue. The transition from successful animal models to human application demands extensive testing and refinement of protocols. Key areas requiring advancement include:
- Developing patient-specific scaffolds that precisely match individual ear anatomy
- Creating reliable methods for nerve integration and sensory function restoration
- Establishing protocols for vascular network formation within the grown tissue
- Ensuring long-term tissue stability and preventing rejection
- Refining surgical techniques for successful implantation
The psychological impact of this technology shouldn’t be underestimated, as living engineered ears could dramatically reduce the emotional burden many patients experience with traditional reconstructive methods. Current approaches often require multiple surgeries and may not achieve optimal aesthetic results, leaving patients feeling self-conscious about their appearance.
Traditional reconstructive surgery typically involves harvesting cartilage from the patient’s ribs, a process that creates additional surgical sites and potential complications. This new approach could eliminate those concerns while providing more natural-looking and potentially better-functioning results. Similar to how artificial intelligence has revolutionized various fields, tissue engineering represents a paradigm shift in reconstructive medicine.
The broader implications of this research extend into multiple medical specialties. Surgeons treating burn victims, cancer patients requiring extensive reconstruction, and individuals born with facial differences could all benefit from these advances. The technology might eventually expand to address other reconstructive needs, potentially transforming how medical professionals approach tissue replacement throughout the body.
As this research progresses, collaboration between Chinese scientists and international medical communities will likely accelerate development and ensure global access to these life-changing treatments. The five-year timeline for clinical trials represents an ambitious but achievable goal, bringing hope to thousands of patients who could benefit from this revolutionary approach to facial reconstruction.
Revolutionary 3D Printing Technology Creates Full-Sized Human Body Parts
Scientists are pushing the boundaries of regenerative medicine with groundbreaking 3D printing technologies that create functional human body parts. I’ve witnessed incredible advancements in this field, particularly with the development of the Integrated Tissue-Organ Printer (ITOP), which represents a quantum leap in bioprinting capabilities.
Advanced Bioprinting Creates Complex Human Tissues
The ITOP system stands out as a game-changing innovation that can print full-sized, cell-laced human body parts complete with internal channels for blood vessel integration. This technology addresses one of the most critical challenges in tissue engineering: ensuring transplanted tissues receive adequate blood supply for survival post-implantation. Engineers have designed these printed structures with sophisticated internal channel systems that allow blood vessels to grow and connect naturally within the tissue.
Scientists are also developing various supporting technologies that complement this advanced bioprinting. These innovations include:
- Mini-organs called organoids that replicate specific organ functions for research and testing
- Organs-on-chips that simulate human organ responses in laboratory settings
- Engineered blood vessels using living scaffold techniques
- 3D-printed bones and muscle tissues with enhanced structural integrity
Living Scaffolds Enable Natural Tissue Integration
The scaffold technology behind these innovations uses biocompatible materials that gradually dissolve as new tissue grows. I find this approach particularly fascinating because it mimics natural tissue development processes. Blood vessel formation occurs organically within these scaffolds, creating networks that support long-term tissue survival.
Current research shows these technologies are rapidly expanding the possibilities for complete organ replacement. While artificial intelligence helps optimize printing parameters, the biological components remain the star of the show. Scientists can now print complex structures that were impossible just a few years ago, including tissues with multiple cell types and integrated vascular systems.
These advancements suggest that full organ replacement may become routine medical practice within the next decade. The combination of ITOP technology with living scaffolds creates a powerful platform for regenerative medicine that could revolutionize how doctors treat organ failure and tissue damage.
Sources:
Futurism – Scientists Successfully Grow a Living Human Ear on a Rat
South China Morning Post – How Chinese scientists cracked the secret of organ regeneration
Science – Reactivation of mammalian regeneration by turning on an evolutionarily disabled gene regulatory network (June 2025)
Science | AAAS – Tissue printer creates lifelike human ear
Live Science – Body parts grown in the lab